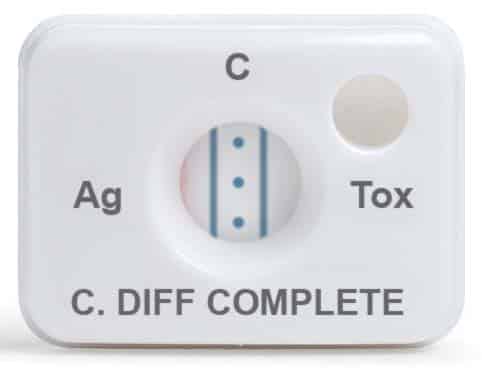
February 2024—TechLab’s C. Diff Quik Chek Complete test is a rapid membrane enzyme immunoassay for the simultaneous detection of Clostridium difficile glutamate dehydrogenase antigen and toxins A and B in a single reaction well. Results are available in less than 30 minutes.
Read More »TechLab to supply ASRs for SARS-CoV-2
January 2021—TechLab has partnered with New River Valley COVID-19 Task Force to expand future COVID-19 testing capacity. Under the agreement, the New River Health District, headquartered in Christianburg, Va., secures access to TechLab’s analyte-specific reagents for SARS-CoV-2 for $7 to $9 per use.
Read More »TechLab partners with New River Health District
Nov. 9, 2020—TechLab has partnered with New River Valley COVID-19 Task Force to expand future COVID-19 testing capacity.
Read More »FDA clears Techlab H. pylori tests
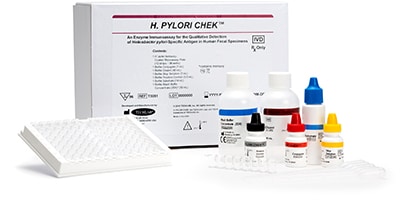
October 2018—Techlab received FDA 510(k) clearance for its H. Pylori Quik Chek and H. Pylori Chek tests. The tests aim to offer quick and reliable detection of Helicobacter pylori–specific antigen in human fecal specimens.
Read More »Techlab gets FDA clearance for H. pylori tests
Aug. 22, 2018—Techlab received FDA 510(k) clearance for its H. Pylori Quik Chek and H. Pylori Chek tests. The tests aim to offer quick and reliable detection of Helicobacter pylori–specific antigen in human fecal specimens. The H. Pylori Quik Chek test is a rapid diagnostic test that detects H. pylori in 30 minutes. The H. Pylori Chek test is a 96-well plate ...
Read More »FDA-cleared test to detect Campylobacter, 4/18
April 2018—Techlab has received FDA clearance for the Campylobacter Quik Chek and the Campylobacter Chek tests to aid the diagnosis of campylobacteriosis. The Campylobacter Quik Chek test is a rapid diagnostic test that detects Campylobacter jejuni and Campylobacter coli in less than 30 minutes.
Read More »Triple parasite screen, 10/17
October 2017—TechLab received FDA clearance for the Tri-Combo Parasite Screen test. The test is an enzyme immunoassay for the simultaneous qualitative detection of Giardia spp., Cryptosporidium spp., and/or E. histolytica antigen in human fecal specimens. It specifically detects pathogenic E. histolytica and does not cross-react with nonpathogenic E. dispar.
Read More »E. histolytica diagnostic test, 8/17
August 2017—Techlab Inc. received FDA clearance for its E. Histolytica Quik Chek test, a rapid diagnostic test that specifically detects pathogenic E. histolytica and does not cross-react with nonpathogenic Entamoeba dispar.
Read More » CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management