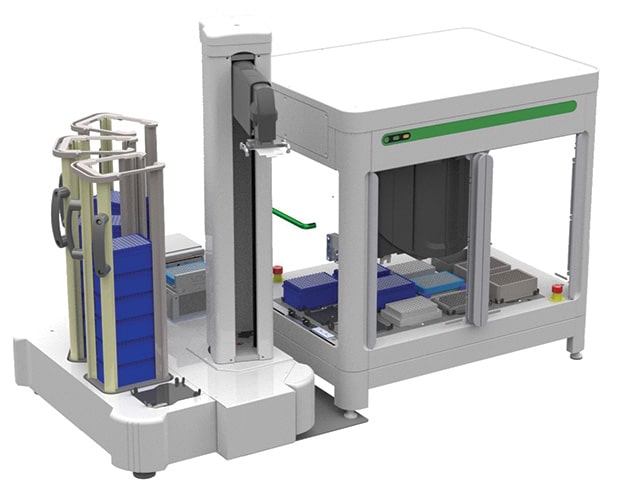
March 2023—PerkinElmer debuted its Zephyr G3 NGS iQ workstation at the Society for Laboratory Automation and Screening conference in San Diego, Feb. 25–March 1. The system enables the automated construction of up to 96 NGS libraries and is composed of a high-performing liquid handler, integrated thermocycler, robotic arm, and deck accessories and peripherals. The workstation is available with an optional cloud-based software to further simplify creating and editing NGS protocols, the company says, by using a single pane of glass interface and developing methods from scientific intent rather than code.
Read More »FDA authorizes PerkinElmer Eonis kit for SMA screening in newborns
January 2023—PerkinElmer announced that the FDA has authorized the marketing of its Eonis SCID-SMA assay kit for in vitro diagnostic use by certified laboratories for the detection of spinal muscular atrophy and severe combined immunodeficiency in newborns. This is the first FDA-authorized assay for SMA screening in newborns in the United States, the company says, and is part of the company’s Eonis platform. The Eonis platform is a robust, flexible system that uses real-time PCR technology to screen for SMA and SCID using a single dried blood spot sample.
Read More »FDA OKs PerkinElmer Eonis kit for SMA screening in newborns
Nov. 16, 2022—PerkinElmer announced that the FDA has authorized the marketing of its Eonis SCID-SMA assay kit for in vitro diagnostic use by certified laboratories for the detection of spinal muscular atrophy and severe combined immunodeficiency in newborns.
Read More »FDA approves T-Cell Select to automate TB testing
Sept. 22, 2022—Oxford Immunotec, a division of PerkinElmer, announced FDA approval of its T-Cell Select reagent kit for automating the company’s T-SPOT.TB test workflow for in vitro diagnostic use by certified laboratories.
Read More »PerkinElmer launches BioQule NGS system
July 2022—PerkinElmer has launched its research use only BioQule NGS system, an automated benchtop solution for next-generation sequencing library preparation of up to eight samples. The system requires as little as 10 ng of starting material per run to create libraries. By incorporating automated thermocycling, integrated quality control through optical quantification, and robust liquid-handling technology in a single device, the company says the system enables researchers to produce high-quality NGS libraries that yield reliable, reproducible results.
Read More »PerkinElmer gets EUA for SARS-CoV-2 respiratory panel, ELISA
November 2021—PerkinElmer announced that the FDA has issued emergency use authorization for its PKamp Respiratory SARS-CoV-2 RT-PCR Panel 1 assay. The RT-PCR test provides simultaneous qualitative detection and differentiation of SARS-CoV-2, influenza A, influenza B, and respiratory syncytial virus isolated from nasopharyngeal swabs, anterior nasal swabs, and midturbinate swabs.
Read More »PerkinElmer SARS-CoV-2 respiratory panel, ELISA get EUA
Oct. 13, 2021—PerkinElmer announced that the FDA has issued emergency use authorization for its PKamp Respiratory SARS-CoV-2 RT-PCR Panel 1 assay.
Read More »PerkinElmer launches solutions to detect SARS-CoV-2 variants
June 2021—PerkinElmer launched two research use only solutions, PKamp VariantDetect SARS-CoV-2 RT-PCR Assay and the next-generation-sequencing–based Nextflex Variant-Seq SARS-CoV-2 Kit. The PKamp VariantDetect SARS-CoV-2 RT-PCR assay can detect mutations associated with B.1.1.7, B.1.351, and P.1 variants. The Nextflex Variant-Seq SARS-CoV-2 WGS workflow can detect all SARS-CoV-2 genetic changes relative to the strain originally identified in Wuhan, China.
Read More »PerkinElmer assay gets EUA for asymptomatic testing
April 2021—PerkinElmer announced that its PerkinElmer New Coronavirus Nucleic Acid Detection Kit received emergency use authorization from the FDA to test individuals without symptoms or other reasons to suspect COVID-19 infection.
Read More »PerkinElmer COVID-19 POC antigen test
March 2021—PerkinElmer launched its PerkinElmer COVID-19 Antigen Test for the qualitative detection of SARS-CoV-2 nucleocapsid protein antigen in nasal or nasopharyngeal swab specimens. The lateral flow immunoassay test can be used to screen or to aid in diagnoses of COVID-19 in asymptomatic or symptomatic individuals. A positive or negative result can be obtained in 15 minutes.
Read More »PerkinElmer acquires Horizon, gets EUA for sample pooling
February 2021—PerkinElmer and Horizon Discovery have reached an agreement in which PerkinElmer will acquire Horizon for about $383 million.
Read More »PerkinElmer to acquire Horizon, gets EUA for sample pooling
Nov. 4, 2020—PerkinElmer and Horizon Discovery have reached an agreement in which PerkinElmer will acquire Horizon for about $383 million.
Read More »PerkinElmer COVID-19 serology test gets EUA
June 2020—PerkinElmer announced that the FDA has provided emergency use authorization for Euroimmun’s Anti-SARS-CoV-2 ELISA (IgG) serology test. Clinical laboratories certified to perform high-complexity tests under CLIA can use this ELISA for the detection of antibodies of the immunoglobulin class G.
Read More »FDA provides EUA to PerkinElmer for COVID-19 serological test
May 7, 2020—PerkinElmer announced that the FDA has provided emergency use authorization for Euroimmun’s Anti-SARS-CoV-2 ELISA (IgG) serology test.
Read More »FDA approves newborn screening test for DMD
January 2020—The FDA authorized marketing of PerkinElmer’s GSP Neonatal Creatine Kinase-MM kit, the first test to aid in newborn screening for Duchenne muscular dystrophy.
Read More »PerkinElmer introduces DNA test
June 2019—PerkinElmer, in collaboration with Helix, launched a genetic screening test called GenePrism: Actionable Insights, which analyzes a subset of 59 medically actionable genes, including BRCA1 and BRCA2, identified by the American College of Medical Genetics and Genomics.
Read More »Euroimmun IFA mosaics cleared with EuroPattern
October 2018—PerkinElmer announced that its Euroimmun ANCA IFA and Europlus Granulocyte Mosaic assays for use with its EuroPattern microscope have received 510(k) clearance from the FDA. The Euroimmun IFA Granulocyte assays are designed as indirect immunofluorescence tests for the qualitative or semiquantitative determination of antineutrophil cytoplasmic antibodies of immunoglobulin class IgG in human serum.
Read More »FDA clears lysosomal storage disorder test
September 2018—PerkinElmer has received FDA 510(k) clearance for its NeoLSD MSMS kit, an in vitro diagnostic that uses tandem mass spectrometry to screen for six lysosomal storage disorders in newborns via a single dried blood spot sample. The kit is intended for the quantitative measurement of the activity of the enzymes acid β-glucocerebrosidase, acid sphingomyelinase, acid α-glucosidase, β-galactocerebrosidase, α-galactosidase A, ...
Read More »Agena, PerkinElmer team up
Aug. 24, 2018—Agena Bioscience announced it has entered into a collaboration with PerkinElmer, incorporating the LabChip GX Touch nucleic acid analyzer for quality assessment and quantitation of DNA in the upfront workflow of Agena’s MassArray system. The companies have focused on targeting ctDNA in oncology liquid biopsy, where the combined systems aim to support a low cost, highly robust, single-day ...
Read More »PerkinElmer, IDG team up for WGS program , 10/17
October 2017—PerkinElmer is collaborating with In-Depth Genomics to support IDG’s whole genome sequencing diagnostic program, which will bring genetic diagnosis to patients who have a wide range of neurological conditions, including orphan disorders, and aims to provide improved diagnoses and treatments.
Read More »PerkinElmer buys Tulip Diagnostics, 4/17
April 2017—PerkinElmer has entered into a definitive agreement to acquire Tulip Diagnostics Private Ltd. Tulip, based in Goa, India, is a large domestic provider of in vitro diagnostic reagents, kits, and instruments to diagnostic labs and government and private health care facilities.
Read More »Fully automated IHC multiplexing kits, 2/17
February 2017—PerkinElmer’s Opal Multicolor Immunohistochemistry products for use with Leica Biosystems’ fully automated Bond RX research staining platform is now available. The kits can visualize up to six biomarkers and a nuclear counterstain on a single tissue section with next-day results.
Read More »PerkinElmer acquires Vanadis, 3/16
March 2016—PerkinElmer has acquired Vanadis Diagnostics. Based in Sweden, Vanadis is developing a novel solution for non-invasive prenatal testing based on digital analysis of cell-free DNA.
Read More »Quantitative pathology imaging system, 2/16
February 2015—PerkinElmer launched its Vectra 3 automated, high-throughput quantitative pathology imaging system. This solution’s new seven-color multiplexing and visualization capabilities are designed to enable pathologists and oncologists conducting research to gain a deeper level of understanding of disease mechanisms related to new cancer immunotherapy approaches.
Read More »PerkinElmer, Covaris partnership, 8/15
PerkinElmer’s Chemagic research instrumentation and kits are now available with the Covaris TruXtrac FFPE DNA MicroTube Kit for Chemagen Technology for DNA/RNA purification powered by Adaptive Focused Acoustics technology. The kit rapidly removes paraffin and rehydrates formalin-fixed, paraffin-embedded tissues in an aqueous buffer. This preparation is optimized to work with PerkinElmer’s automated magnetic bead-based DNA/RNA separation technology for low-, medium-, and high-throughput solutions.
Read More »Multilabel slide scanner, 5/14
May 2014—PerkinElmer launched the Lamina multilabel slide scanner at the USCAP 2014 Annual Meeting. The scanner is a high-throughput imaging system for research pathologists in a larger research facility to study protein expression and the relationships between disease markers in formalin-fixed, paraffin-embedded tissue sections.
Read More »In vivo imaging system, 3/13:76
PerkinElmer’s Ivis Lumina series III benchtop platform is for oncology, infectious disease, and drug discovery and efficacy research. The system integrates bioluminescence and full spectral fluorescence in vivo imaging technologies, providing an expandable and sensitive benchtop imaging platform.
Read More »PerkinElmer, Verinata to expand access to prenatal test, 3/13:75
PerkinElmer and Verinata Health announced a strategic agreement for expanding access to Verinata’s Verifi test, a comprehensive noninvasive prenatal test (NIPT) for high-risk pregnancies. The Verifi test, performed at Verinata’s CLIA-certified California laboratory, uses a single maternal blood draw at as early as 10 weeks of pregnancy to detect multiple fetal chromosomal aneuploidies. Under terms of the collaboration, PerkinElmer will serve as an exclusive Verinata partner in joint sales and marketing of the Verifi test.
Read More » CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management