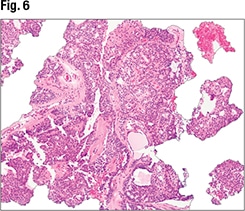
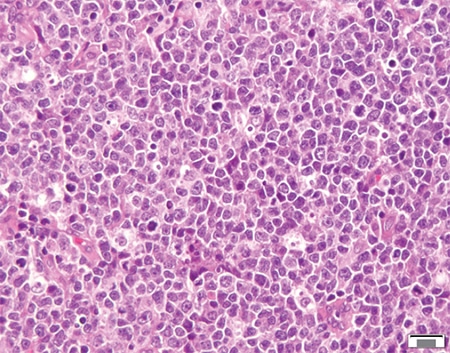
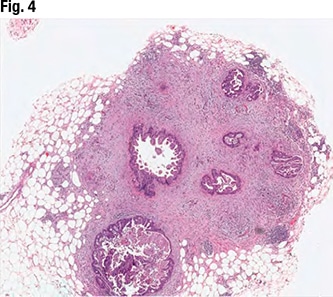
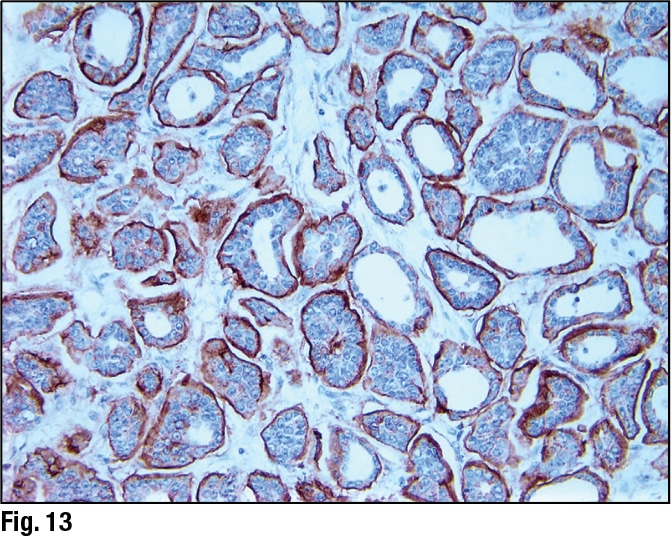
January 2024—Much has been said and written about scoring HER2-low breast cancer, and it has its difficulties. But there are steps and tools to support scoring, and Savitri Krishnamurthy, MD, last fall shined a light on them and several HER2-low breast cancer-related studies.
Read More »Fast or comprehensive? Lab offers both for NSCLC
October 2023—For molecular testing in oncology, the choice is often fast or slow. PCR-based platforms are rapid, and comprehensive genomic profiling by next-generation sequencing is slower, and each has its pros and cons.
Read More »AMP case report: Identification of multiple germline cancer predisposing gene variants in a single patient during tumor sequencing analysis
October 2023—Next-generation sequencing of tumor tissue has important implications in solid and hematologic malignancies because it can identify genomic variants that provide diagnostic, prognostic, and predictive information to guide clinical management. Variants identified on tumor sequencing can be classified as somatic (acquired after conception) or inherited through germline.
Read More »Low level limbo in HER2 breast cancer
August 2023—Seemingly channeling the inspiration of Magritte and his famous pipe, pathologists are painting a new picture of what has long been an everyday object in their own world: HER2. To paraphrase the master: Ceci n’est pas facile. For years, HER2 testing in breast cancer has seemed self-evident, ever since the HER2-targeted therapy trastuzumab and its companion diagnostic arrived on the scene a quarter of a century ago. Pathologists became comfortable using immunohistochemistry to identify 3+ cases and turning to in situ hybridization techniques to sort through less obvious ones. But early last summer, a variant of the drug, trastuzumab-deruxtecan (T-DXd), shook up that routine. When researchers presented results from the Destiny-Breast04 study at the 2022 ASCO annual meeting, showing that T-DXd significantly improves survival in so-called HER2-low metastatic breast cancer, attendees responded with a minutes-long standing ovation. They then returned from the meeting like evangelicals from the revival tent.
Read More »Cytopathology in focus: How to approach cytology of unknown primary
August 2023—We discuss in this article a common problem that all cytopathologists come across frequently in their practice: tumors of unknown primary origin involving body fluids and other sites. Metastatic tumor cells can disseminate and colonize discontinuous secondary body sites.1 Such tumor metastases may be the patient’s initial presenting complaint to a family physician for deep-seated tumor primaries such as ovaries, pancreas, liver, and certain non-obstructive gastrointestinal tumors.
Read More »Cytopathology in Focus: Lung cytopathology reporting: WHO system and cases
August 2023—Accurate and timely diagnosis is the cornerstone of effective patient care, particularly in the field of pulmonary pathology. To address the challenges health care professionals face in diagnosing and reporting respiratory conditions, the International Academy of Cytology, together with the International Agency for Research on Cancer, recently developed the World Health Organization Reporting System for Lung Cytopathology
Read More »AMP case report: A germline GATA2 c.121C>G (p.P41A) variant in a patient with an unusual acute promyelocytic leukemia
July 2023—A germline GATA2 c.121C>G (p.P41A) variant in a patient with an unusual acute promyelocytic leukemia CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Emory University School of Medicine. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Read More »Appendiceal lesions: features, subtypes, patterns
June 2023—Although goblet cell adenocarcinoma can label for neuroendocrine markers, it behaves as an adenocarcinoma and is staged as such. And it’s important to distinguish goblet cell adenocarcinoma from tubular neuroendocrine tumor, a rare subtype of neuroendocrine tumor.
Read More »Colorectal cancer next on HER2 horizon
May 2023—Behold the common coin. Note its two sides, its easy flippability. Here is Joseph Pizzolato, MD, with the first coin toss. Given the expanded use of biomarkers with a variety of tumors, and constantly evolving assays, how hard is it for medical oncologists to navigate testing? “It’s not difficult at all now,” says a cheerful Dr. Pizzolato, medical director of the comprehensive therapeutic unit of Sylvester Comprehensive Cancer Center, University of Miami Health System, as well as medical director of the Aventura satellite at Sylvester. With third-party companies integrating test ordering directly into electronic medical records, he adds, “It’s getting even easier to order tests and see the results.” Agreed, says his colleague Rhonda Yantiss, MD, director of surgical pathology, Department of Pathology and Laboratory Medicine, University of Miami Miller School of Medicine. And therein lies the problem. “It’s kind of a mess,” she says. In practice, precision medicine is becoming both more and less precise.
Read More »Growing pains put gene panels in a pinch
April 2023—After years of excitement and scientific breakthroughs, the use of molecular testing to guide cancer therapeutics finally is coming into its own. Unfortunately, it appears to have landed in the wrong place at the right time. That place is a lonely spot, surrounded by gaps in economics and coverage, as well as knowledge, guidelines, ordering patterns, turnaround times, reporting, and the like. So plentiful are the gaps that, put together, they could form a vast, inhospitable space, a veritable Colorado Plateau, with molecular testing as a majestic, enticing but remote rocky pinnacle in the middle. Think Monument Valley. It’s worth the trek. The evidence in support of genomic profiling continues to grow. Simply put, “Patients with the right markers who get the right drugs do better,” says Neal Lindeman, MD, vice chair, laboratory medicine and molecular pathology, Department of Pathology and Laboratory Medicine, Weill Cornell Medicine/New York Presbyterian Hospital. But as numerous studies are showing, the lag in testing is growing as well.
Read More »Ancillary tests in gynecologic pathology—what and why
April 2023—With serous ovarian carcinomas and other gynecologic tumors, it’s molecular testing that increasingly sets the treatment course. “As pathologists, it’s exciting to us,” said Sadia Sayeed, MD, speaking at CAP22. “We’re learning that the immunostains we’re interpreting have greater implications than we ever thought they could.”
Read More »A start at standardizing neoplastic cellularity assessment
April 2023—Molecular testing guidelines say that the neoplastic cell content of each specimen should be assessed, but there are no formal recommendations or guidelines on how to do the assessment.
Read More »New paths through hematologic neoplasms
March 2023—Updated classifications for hematologic neoplasms are here. Let the complications continue. As with other specialties, hematopathology has been absorbing advances gleaned from molecular and genetic data. In some cases, this can tilt diagnosis away from primarily immunophenotypic approaches. It might lead to splits in what was formerly a single entity. On occasion, it might suggest further testing options that could be of value to patients now, or possibly at a date down the road. Or it might just leave pathologists and their clinical colleagues peering at a lack of data, knowing they have to make decisions nonetheless. Two groups—the World Health Organization and the International Consensus Classification—have put forth classifications to help physicians sort through the complexities. The WHO published a beta version of the fifth edition of its Classification of Haematolymphoid Tumours in July 2022.
Read More »Spotlight on ancillary tests in endometrial cancer
March 2023—With endometrial carcinomas and other gynecologic tumors, molecular testing matters, and not only at the diagnostic stage. “We’re rapidly evolving,” said Leslie M. Randall, MD, MAS, division director of gynecologic oncology, Virginia Commonwealth University Health, speaking at CAP22.
Read More »Breast cancer biomarkers, classic and new
February 2023—Like a thriving expat, Deborah Dillon, MD, is comfortable moving within worlds both old and new. Specifically, as a breast and molecular pathologist at Brigham and Women’s Hospital, she appreciates the biomarkers she and her colleagues grew up with, so to speak, as well as those that are part of a more recently arrived-at scenery. Not everyone finds both worlds equally riveting. “A lot of people are much more interested in, and excited by, new markers,” she says. “When I talk to people from pharma, this is what they want to hear about.” So do many pathologists, oncologists, and patients—new markers and new therapies have a way of updating hopes. Dr. Dillon understands the persistent thrill of the new, why people want her to talk the language of PIK3CA, PARP inhibitors, MMR, NTRK fusions, ESR1, and the like. But as an in-demand speaker as well as in a recent interview with CAP TODAY, she also advocates for making the old—the longstanding trinity of ER, PR, and HER2—seem new again.
Read More »Evaluating post-treatment breast specimens
January 2023—Laura Esserman, MD, MBA, can still recall her Eureka moment. She had just seen a talk on residual cancer burden by pathologist W. Fraser Symmans, MB.ChB, a pioneer in the field. “When I saw Fraser present this,” says Dr. Esserman, director, University of California San Francisco Breast Care Center, “I knew immediately that MRI would work and that residual cancer burden would complement it. MRI was basically a snapshot of RCB over time. I realized that we had to institute RCB—we had to standardize our approach.” Until then, she and her colleagues across the I-SPY trial sites relied on individual pathologist assessment for each case. The pathologic complete response rate, or pCR, hovered at about 34 percent. That insight was soon followed by another. Intrigued by what she heard, Dr. Esserman and her pathologist colleagues from all the I-SPY sites traveled to MD Anderson, where Dr. Symmans helped develop the residual cancer burden system, for training.
Read More »No time to wait: How rapid NGS changed cancer care
November 2022—Rapid next-generation sequencing in a community hospital setting, performed by histotechnologists and interpreted by anatomic pathologists, is possible and paying off, and it “makes the pathologist a much more meaningful part of the precision oncology team,” says Brandon Sheffield, MD, of the Department of Laboratory Medicine, William Osler Health System, Brampton/Etobicoke, Ontario. “It has changed practice at our hospitals,” he says.
Read More »Scoring HER2 expression across the full spectrum
October 2022—HER2-low breast cancers are now of greater clinical interest, given Enhertu’s recent approval for use in treating such cancers. How to achieve accurate and reproducible results in scoring HER2-low tumors was at the center of a CAP TODAY webinar on new perspectives on the full spectrum of HER2 expression in breast cancer.
Read More »A practical approach to borderline melanocytic neoplasms
August 2022—In cases of borderline melanocytic neoplasms, which have overlapping histopathologic features of benign and melanocytic lesions, additional immunohistochemical studies sometimes help to differentiate the two. But a subset of lesions will show overlapping features.
Read More »Breast cancer breakthrough sparks HER2 quest
June 2022—The latest advance in breast cancer treatment is a big one—the promising antibody drug conjugate fam-trastuzumab deruxtecan-nxki, or T-DXd (Enhertu). The drug was granted breakthrough therapy designation this spring for patients with HER2-low metastatic breast cancer, and the drug and trial on which the decision was based were the focus of the plenary session at the ASCO annual meeting in early June. “This drug in particular is a variant of a drug we are all very familiar with—Herceptin, or trastuzumab,” says David Rimm, MD, PhD, the Anthony N. Brady professor of pathology, professor of medicine (oncology), director of the translational pathology and Yale pathology tissue services, and director of the physician scientist training program in pathology, Department of Pathology, Yale University School of Medicine. Also familiar: the IHC test to determine eligibility for the drug, a companion diagnostic developed decades ago. But that’s where easy familiarity ends.
Read More »Fluid cytology—key features and ancillary testing
June 2022—What to look for in serous fluid cytology is what Eva M. Wojcik, MD, of Loyola University in Chicago, and Xiaoyin “Sara” Jiang, MD, of Duke Health, set forth in their CAP21 session last year.
Read More »AMP case report: ETV6/FLT3 fusion gene detected in a patient with T-cell lymphoblastic lymphoma
April 2022—Genetic alterations of the gene FLT3, especially internal tandem duplications in the juxtamembrane domain and point mutations in the tyrosine kinase domain, are commonly seen in patients with newly diagnosed myeloid leukemias. However, chromosome rearrangements involving the FLT3 gene are extremely rare in hematologic malignancies. The FLT3 gene has only a few known partner genes, including the gene ETV6, which encodes a transcriptional repressor. ETV6 has a wide variety of translocation partner genes, several of which are tyrosine kinase genes.
Close-up on common diagnoses in core biopsies
March 2022—Papillary and fibroepithelial lesions of the breast were the focus of a talk given by Xiaoxian (Bill) Li, MD, PhD, in a CAP21 session on common but challenging diagnoses in breast core biopsies.
Read More »Molecular oncology tumor board: The curious cases of medullary thyroid cancer
March 2022—Most thyroid cancer cases are straightforward, but a small percentage are not. In a CAP21 molecular oncology tumor board session, Justin Bishop, MD, and Saad Khan, MD, spotlighted one of the latter.
Read More »Gastric intestinal metaplasia—the need to classify and how
February 2022—How to classify gastric intestinal metaplasia, when to classify it, and the implications of a GIM diagnosis were the focus of a CAP21 presentation in a session on advances in gastric neoplasms. The big question, said presenter Namrata Setia, MD, is, “Why are we suddenly talking about classifying intestinal metaplasia in the stomach?
Read More »Molecular oncology tumor board: Pathologist, oncologist dip into head and neck case
February 2022—Up first in a CAP21 molecular oncology tumor board session was an unusual case of head and neck cancer, one that raises questions about what salivary duct carcinoma is and the role of next-generation sequencing. Justin Bishop, MD, the Jane B. and Edwin P. Jenevein, MD, distinguished chair in pathology and professor and chief of anatomic pathology, UT Southwestern, along with Saad Khan, MD, assistant professor of medicine (oncology) at Stanford, teamed up to present the case, along with a second case that will be reported in the March issue. (Dr. Khan practiced at one time at UT Southwestern, so this wasn’t their first tumor board together.)
Read More »AMP case report: A case of a rare myeloid neoplasm presenting with features mimicking primary myelofibrosis
February 2022—Myeloproliferative neoplasms (MPNs) are a group of clonal hematopoietic stem cell disorders characterized by increased proliferation of myeloid cells of one or more lineage. The most common MPNs include chronic myeloid leukemia (CML), polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). CML is defined by the BCR-ABL1 fusion, which typically results from the t(9;22)(q34;q11.2) rearrangement.
Read More »MMR, MSI testing guideline nears finish line
December 2021—No single assay can capture all cancer patients with DNA mismatch repair deficiency, and in determining a patient’s eligibility for immune checkpoint inhibitor therapy, assays for MMR deficiency, microsatellite instability, and tumor mutation burden should not be considered interchangeable.
Read More »DELFI approach as ‘pretest’ in early cancer detection
December 2021—A cost-effective liquid biopsy focused on analyzing genomewide fragmentation profiles in cell-free DNA has been shown in proof-of-concept studies to detect early-stage lung and other cancers.
Read More »A sizable shift in CNS tumor classification
November 2021—Much has changed since the last WHO classification of central nervous system tumors was published five years ago. Case in point: When the group of authors met in Utrecht, the Netherlands, in late 2019, everyone anticipated two more WHO meetings in Europe to work further on the 2021 classification. Arie Perry, MD, a coauthor on both classifications, says the group photo was cheery. “Everybody was smiling.” The later trips to Europe were canceled because of the pandemic and the group met instead by Zoom. “Now everybody looked grumpy,” he says of a screen shot. Fortunately, “We got everything done, even if it wasn’t quite as pleasant,” says Dr. Perry, professor of pathology and neurological surgery, Department of Pathology, Division of Neuropathology, University of California, San Francisco. The result is the latest WHO classification, which offers dramatic changes of its own. “I’m really excited about the new WHO,” he says. “At first it takes a little getting used to”—like, say, a face mask—“but I think it’s another major advance, just like we had last time.”
Read More »AMP case report: A patient with an unexpected cancer predisposition syndrome—somatic tumor mutation testing and germline mutation testing complement each other
November 2021—Molecular analysis of advanced stage tumors has become the gold standard for identifying potential targetable mutations with high sensitivity, even in limited size tissue samples. However, when only tumor tissue is sequenced, it is difficult to differentiate between somatic mutations in the tumor cells versus constitutional (germline) mutations.
Read More »Up close on clonal hematopoiesis in cfDNA testing
October 2021—Clonal hematopoiesis is a significant biological phenomenon and denotes presence of mutations in bone marrow stem cells in the absence of a hematologic malignancy.
Read More »Molecular or morphology? Challenges in pathologic diagnoses
October 2021—Recent molecular genetic advances have dramatically expanded diagnostic options, thus revolutionizing the diagnosis of many tumor types, especially those of soft tissue and bone. Advances in the discovery of molecular alterations underlying neoplastic pathogenesis have also provided insights into novel therapeutic targets and prognostic biomarkers. These improvements have led to the reclassification of a growing list of previously established tumor types, resulting in significant challenges for practicing pathologists, as exemplified herein.
Read More »Detecting myeloid malignancy minimal residual disease
October 2021—Detecting leukemic cells for post-treatment monitoring in normal karyotype acute myeloid leukemia is challenging, but new approaches to minimal residual disease monitoring may make it increasingly possible in the clinical laboratory, David Wu, MD, PhD, said in an AMP webinar he presented recently on myeloid malignancy minimal residual disease detection.
Read More »Gastric HER2, hsALK to join monitored PT list
September 2021—Beginning next year, CAP-accredited laboratories that perform HER2 immunohistochemistry in gastroesophageal adenocarcinoma or highly sensitive (hs) ALK in non-small cell lung cancer will be required to enroll in proficiency testing for those analytes.
Read More »For MammaPrint and BluePrint, the long-term view
September 2021—The latest data on the use of two genomic assays in early-stage breast cancer and at the University of Rochester Medical Center as the pandemic set in were reported in a CAP TODAY webinar presented by William Audeh, MD, and David G. Hicks, MD. Dr. Audeh, medical oncologist and chief medical officer of Agendia, developer of MammaPrint and BluePrint, presented the long-term follow-up results of the MINDACT trial and an age-related analysis, as well as new data on MammaPrint’s use in endocrine therapy decisions.
Read More »A few years in, a new picture for liquid biopsy
July 2021—Liquid biopsy has entered a more confident era, with two FDA-approved next-generation sequencing assays for comprehensive tumor mutation profiling, evidence of its clinical utility, and broadened patient access.
Read More »Lymphocytosis: distinguishing benign from malignant
May 2021—How to distinguish “reactive” and “nonreactive” benign lymphocytosis from malignant lymphocytosis, and between benign and malignant large granular lymphocytosis, is how Kyle Bradley, MD, of Emory University, opened his talk in a CAP20 virtual session last fall.
Read More »NGS in more labs? IFCC group aims to ease the way
May 2021—When it comes to next-generation sequencing, don’t count out community hospital labs, especially as black-box solutions come on the market. That’s the hope of members of an International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) working group that aims to help clinical labs develop in-house NGS programs. Large-scale genomic testing won’t be necessary or practical at the community hospital level. But hospital-based genomic testing programs should set out to meet the NCCN guideline targets and provide testing for which a wide range of sample input and quality can be accepted, says Robyn Sussman, PhD, a member of the IFCC working group and molecular development assistant director, Penn Precision and Computational Diagnostics, University of Pennsylvania Perelman School of Medicine.
Read More »From manual to staging system—AJCC’s new path
April 2021—In the continuum of cancer care, diagnostic specialties such as ours play critical roles. We touch virtually all aspects of cancer care through cancer diagnostics, the use of molecular studies in treatment and as predictive markers, and our key role in anatomic pathologic staging. The widespread use of the CAP cancer protocols has improved cancer reporting by standardizing format and terminology while also incorporating the concept of human factors engineering and standards for developing high-quality clinical practice guidelines.
Read More »AMP case report: Adult B-lymphoblastic leukemia/lymphoma, BCR-ABL1-like
April 2021—A 71-year-old female with a history of asthma and hypertension initially presented to her local hospital complaining of shortness of breath. She was found to be pancytopenic with severe anemia (hemoglobin 5 g/dL). She was subsequently transferred to a tertiary care facility for further evaluation. Bone marrow biopsy revealed a hypercellular marrow composed of 72 percent blasts. Flow cytometric analysis revealed a B-lymphoblast immunophenotype with expression of CD34, dim CD45, CD19, CD79a, CD22, HLA-DR, TDT, CD200, CD33, and dim CD13.
Read More »AMP case report: A CLL/SLL case with distinctive molecular and cytogenetic changes during different stages of disease progression
March 2021—Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is one of the most common lymphoproliferative diseases. It is a CD5-positive B-cell neoplasm of monomorphic small mature B cells. One of the characteristics of CLL/SLL is its heterogeneity, not only among individuals but also within individual patients.1 The cytogenetic and molecular variants are dynamic during disease progression and in response to targeted therapies.
Read More »Markers, methods remake the NSCLC map
February 2021—Absorbing new biomarkers into lung cancer workups makes for a complicated diplomacy. How best to balance so many rivals? Does it make the most sense for laboratories to try to do everything at once, a full-court press involving next-generation sequencing panels? Or is it more practical to add a new marker only as a new targeted therapy receives approval? Where do RNA-based assays fit in? What about IHC? When do you make the switch? Or do you? And how best to handle cell-free DNA tests (which seem to be the rogue states in all this)? How do you weight external factors, such as reimbursement, existing equipment and capital expenditures, and physician expertise? Driving this all are medical breakthroughs. As with all forms of statecraft, the latest incident can change everything. For lung cancer, the most recent advance comes from the ADAURA trial, which showed a significant benefit of using osimertinib to treat stage IB to IIIA EGFR-mutation positive non-small-cell lung cancer.
Read More »Primary HPV screen only? Experts warn of risks
January 2021—It’s a watershed moment when an influential standard-setting organization, the American Cancer Society, announces that a test widely used for decades should be supplanted—especially when the test is the linchpin of the most successful cancer screening program in U.S. history.
Read More »AMP case report: Role of lymphoma sequencing panel in diagnosis of pediatric-type follicular lymphoma
November 2020—Pediatric-type follicular lymphoma (PTFL) is a rare form of lymphoma that was recognized as a new diagnostic entity in the revised 2016 WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. The classic features of PTFL include male predominance, localized stage I lymphadenopathy, blastoid morphology, high proliferation index, and exceedingly good response rate to local excision.
Read More »Why do universal HRD testing in ovarian cancer?
September 2020—Genetic testing in ovarian cancer has a therapeutic implication that will aid in developing a treatment plan, and it is pathologists who should take the lead in creating the testing protocol, said Samuel Caughron, MD, pathologist, president, and CEO of MAWD Pathology Group, in a recent CAP TODAY webinar. Dr. Caughron explained the rationale for universal homologous recombination deficiency testing in patients with advanced ovarian cancer. The webinar, made possible by a medical sponsorship from AstraZeneca, is at <a href="https://www.captodayonline.com">www.captodayonline.com</a>.
Read More »Targeting immune signaling checkpoints in AML
September 2020—Acute myeloid leukemia was one of the first diseases for which T cells were incorporated into the therapeutic paradigm, in the form of allogeneic stem cell transplant and donor lymphocyte infusion. Why then are there no approved immune therapies, or more specifically checkpoint inhibitors, for this T-cell–sensitive disease?
Read More »Tumor budding assessment in CRC: why and how
August 2020—Tumor budding is a robust prognostic marker that should be reported at least in pT1 and stage II colorectal carcinomas and taken into account with other risk factors. Further evidence is needed for tumor budding assessment in specimens taken after neoadjuvant therapy, says Heather Dawson, MD, senior staff GI pathologist at the Institute of Pathology, University of Bern in Switzerland.
Read More »AMP case report: Burkitt-like lymphoma with 11q aberration
August 2020—Burkitt-like lymphoma with 11q aberration (BLL-11q) is a new provisional entity in the revised 2016 WHO classification of hematopoietic and lymphoid tumors.1 It refers to a subset of high-grade B-cell lymphomas that resemble Burkitt lymphoma with similar morphology, phenotype, and gene expression profiling, but lack MYC gene rearrangements. Instead, these lymphomas carry chromosome 11q proximal gains and telomeric losses, suggesting co-dysregulation of oncogenes and tumor suppressor genes.
Read More »In CRC, distinguishing tumor deposit from lymph node
July 2020—When patients who have colorectal cancer surgery at another institution seek further care at Beth Israel Deaconess Medical Center in Boston, the Beth Israel pathologists routinely request the original slides. Raul S. Gonzalez, MD, a gastrointestinal pathologist at Beth Israel, says he usually agrees with everything the outside pathologist reports. But if there are differences, lymph nodes versus tumor deposits is one place where he might disagree.
Read More »Predicting response to therapy with BH3 profiling
June 2020—Precision medicine in oncology, which today is nearly universally about genetics, needs to move beyond omics and static approaches, Anthony Letai, MD, PhD, professor of medicine at Dana-Farber Cancer Institute and Harvard Medical School, said at last year’s meeting of the Association for Molecular Pathology. Dr. Letai reported how his laboratory uses dynamic BH3 profiling, a novel assay that detects BCL2 protein dependence in cancer cells and measures changes in their apoptotic priming, to predict clinical response to therapy. He and others call it functional precision medicine.
Read More »In NSCLC, biomarker testing rates fall short
June 2020—Testing rates for actionable biomarkers in metastatic non-small cell lung cancer patients are below where they should be, and the overlap of PD-L1 expression with genomic targets causes confusion for oncologists and patients, said Geoffrey R. Oxnard, MD, oncologist at Dana-Farber Cancer Institute and associate professor of medicine at Harvard Medical School, in a recent CAP TODAY webinar.
Read More »ER/PgR guideline hones approach to ER-low positives
April 2020—The CAP and the American Society of Clinical Oncology released two years ago a focused update of their clinical practice guideline for HER2 testing for breast cancer, following an update in 2013. Read more.
Read More »PD-L1 testing in triple-negative breast cancer: Post hoc IMpassion130 substudy evaluates PD-L1 IHC assay performance
April 2020—IMpassion130 was the first phase three trial to demonstrate a clinical benefit of cancer immunotherapy in patients with PD-L1-positive metastatic triple-negative breast cancer, and based on the data, atezolizumab plus nab-paclitaxel is approved for this indication. In the trial, the Ventana SP142 PD-L1 assay with a one percent or greater cutoff was used to evaluate PD-L1 expression in immune cells. But questions remained about how to best identify patients who could benefit from the drug combination, Hope S. Rugo, MD, professor of medicine and director of breast oncology and clinical trials education at the University of California San Francisco Comprehensive Cancer Center, said in a CAP TODAY webinar last November.
Read More »Path to importance of PD-L1 status in breast cancer
June 2019—New data support testing patients for their PD-L1 immune cell status when they are diagnosed with metastatic or unresectable locally advanced triple-negative breast cancer to determine if they might benefit from a checkpoint inhibitor.
Read More »Quantitative image analysis: In guideline, preliminary rules for pathology’s third revolution
April 2019—With the release in January of a new guideline for quantitative image analysis of HER2 immunohistochemistry for breast cancer, the CAP believes it is filling a gap and blazing a trail for the profession. In setting evidence-based standards, the guideline provides background and details about the quantitative image analysis (QIA) process and the data and metadata it generates. The guideline will help facilitate pathology’s increasing use of not only digital pathology but also artificial intelligence, says Marilyn Bui, MD, PhD, chair of the CAP expert panel for QIA of HER2 IHC. “This is not just another guideline. It is a milestone for pathologists.”
Read More »Apocrine breast cancer, ESR1 mutations at center of tumor board review
February 2019—Two breast cases—one of apocrine carcinoma and androgen receptor overexpression and another of metastatic ER-positive cancer and an ESR1 mutation—were the focus of a molecular oncology tumor board session at CAP18.
Read More »Small groups, big answers in HER2 testing
July 2018—Take the new ASCO/CAP guideline for HER2 testing. Since the first groundbreaking joint guideline appeared 11 years ago, the authors have made a habit of addressing cases that flummox pathologists, medical oncologists, and patients. Now, in 2018, they have clarified the diagnostic approach to in situ hybridization groups two, three, and four, rare cases that nonetheless cause an outsized share of headaches and worries. It also clarifies language from the 2013 guideline that had sent some labs astray, and it addresses the use of multiple alternative chromosome 17 probe assays. The previous guidelines turned out to be tough acts to follow—a bit like following Sean Connery in the role of James Bond—even as the new one benefits from new data.
Read More »Core needle biopsy of the breast: cases and cautions
June 2018—With core needle biopsies of the breast, if something looks like an epithelial malignancy, ask yourself: Is it really a carcinoma? If it is a carcinoma, ask yourself if it is a primary breast carcinoma.
Read More »LCIS variants and DCIS: tips on telling them apart
April 2018—DCIS or LCIS? Making the distinction can be difficult in some cases. Stuart J. Schnitt, MD, in a session at CAP17 on ancillary testing in breast pathology, delineated the reasons and provided tips, including the role of E-cadherin immunostains to help in this distinction. The cells of DCIS typically show strong membrane staining for E-cadherin while the cells of LCIS are typically E-cadherin negative. But among the tips: If an in situ lesion is E-cadherin positive, it doesn’t automatically mean it’s ductal carcinoma in situ. As he demonstrated in several cases, the lesion could be lobular carcinoma in situ with aberrant E-cadherin immunostaining.
Read More »Bladder cancer preps for its star turn
February 2016—A streak of sibling rivalry emerges when experts ponder progress in the field of bladder cancer. Whether it’s new markers or therapies, funding or advocacy, advances have come slowly, and the disease has long labored in the shadow of others. “Urologic malignancies in general lag behind, compared to breast cancer and other tumor types, like colon and lung, where we’ve been envious for a while,” says George Netto, MD, professor of pathology, urology, and oncology and director of surgical pathology molecular diagnostics, Johns Hopkins University School of Medicine.
Read More »With DCIS, where does the real risk lie?
December 2015—When a pathologist makes a diagnosis of DCIS, few people greet the news happily. Not patients, not surgeons, not radiation oncologists. Depending on the particulars of the case, pathologists might also feel cheerless. Typically, the only winners are uncertainty and its sidekick, fear.
Read More »Twists and turns in biomarker exploration
August 2015—Forget the lone tree falling down, unnoticed and thus possibly soundless, in the forest. For pathologists and medical oncologists, the more meaningful philosophical question involves breast cancer biomarkers. If a biomarker looks promising in research, will its impact be felt in clinical practice?
Read More »Laying worries to rest over breast biopsy discord
May 2015—With the regularity of a pension fiscal crisis they appear: one study or another, in various journals, pointing out discrepancies in pathology findings. Editorials appear, the news jumps to the lay press, and suddenly the conversation feels hijacked.
Read More »Breast cancer answers, short and long
February 2014—When it comes to breast cancer, medical oncologists have two “wish lists” for their pathologist colleagues. Here’s the short list of test results they need when they sit down with a patient, courtesy of Melody Cobleigh, MD. “ER, PR, HER2,” says Dr. Cobleigh, professor of medicine and the Brian Piccolo Chair for Cancer Research, Rush University Medical Center, Chicago. It’s a direct, unassailable answer. But so, too, is saying that the assassination of the Archduke Ferdinand caused World War I.
Read More »HPV a game changer in head, neck tumors
December 2013—Not that any cancer is ever “easy,” but until relatively recently, the culprit in head and neck squamous cell carcinomas was clear. The vast majority were caused by “smoking, smoking, and smoking,” says William Westra, MD, professor of pathology, oncology, and otolaryngology/head and neck surgery, and associate director, surgical pathology, The Johns Hopkins Medical Institutions. Call this HNSCC’s antediluvian era.
Read More »New guideline takes on tough HER2 cases
October 2013—In HER2 testing for breast cancer, the term “equivocal” verges on being a four-letter word. If the patient has a clearly positive test result, therapies targeting HER2 become a treatment option, and a highly successful one at that. If the result is clearly negative, HER2-targeting drugs are off the table; the patient isn’t expected to benefit from the drugs, which are expensive and can be cardio- toxic.
Read More » CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management