March 2024—Bio-Rad Laboratories has published the third workbook in a P.A.C.E.-approved series designed to provide lab professionals with the training and education needed to maintain reliable test results. “Quality Control Process Optimization with Cost of Quality Application” focuses on a practical approach of cost of quality to the laboratory workflow process of quality control. After using the workbook, laboratorians will be able to construct a basis worksheet to calculate the failure costs of a selected failed laboratory process.
Cardiac Advance now compatible with Beckman instruments
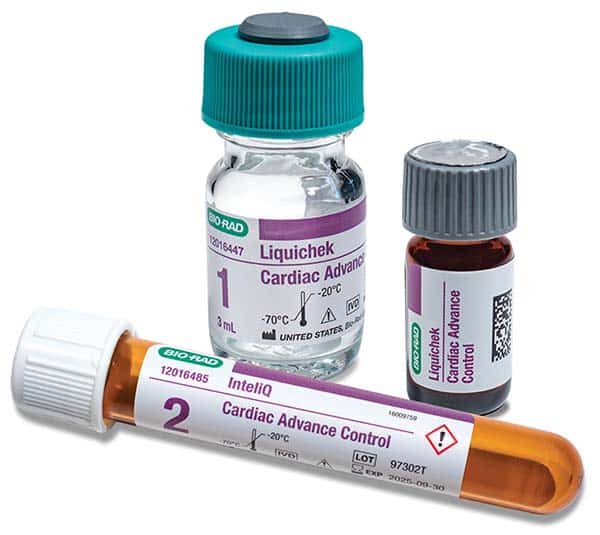
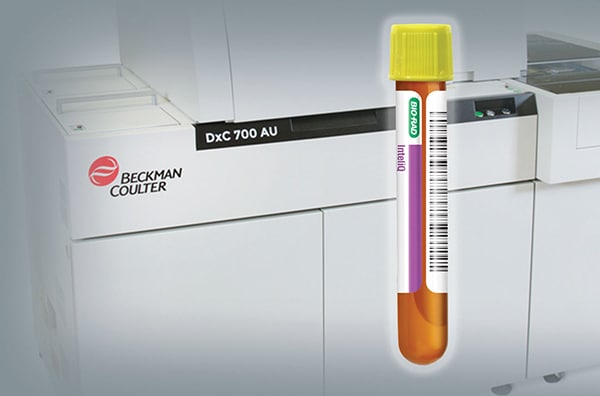
December 2023—Bio-Rad Laboratories has announced the expanded compatibility of its cardiac control, Cardiac Advance, to include Beckman Coulter instruments. The next-generation control is optimized with troponin I and troponin T targets near the limit of instrument detection and contains 10 of the most tested cardiac analytes, including troponin, CK-MB, BNP/proBNP, and myoglobin. Cardiac Advance is available in multiple formats, including the Liquicheck and InteliQ human serum-based controls.
Read More »Bio-Rad launches second installment in QC workbook series
August 2023—Bio-Rad Laboratories has published the second workbook in a series that offers Professional Acknowledgement for Continuing Education credits. The second installment, titled “Laboratory Quality Control Materials,” focuses on identifying proper QC materials and using and handling them appropriately to help monitor QC testing procedures that produce high-quality patient results. Lab professionals can receive a certificate after successfully completing a short exam at the end of the workbook and earn 2.0 contact hours.
Read More »Bio-Rad launches Cardiac Advance quality controls
May 2023—Bio-Rad Laboratories has launched Cardiac Advance, a four-level multianalyte cardiac quality control for high-sensitivity troponin testing.
Read More »Bio-Rad launches Cardiac Advance quality controls
April 18, 2023–Bio-Rad Laboratories has launched Cardiac Advance, a four-level multianalyte cardiac quality control for high-sensitivity troponin testing.
Read More »Bio-Rad, Element Biosciences to deliver RNA sequencing workflow
March 2023—Bio-Rad Laboratories and Element Biosciences announced a partnership to demonstrate the capabilities of the Bio-Rad Sequoia RNA sequencing library preparation portfolio on the Element Aviti benchtop sequencer.
Read More »Bio-Rad launches real-time PCR system
March 2023—Bio-Rad launched its CFX Opus Deepwell Dx real-time PCR system, an open platform for developing in vitro diagnostic assays. The system multiplexes up to five targets and allows reaction volumes up to 125 µL in a 96-well format for quantitative PCR diagnostic assays. CFX Maestro Dx SE software provides data management and analysis tools.
Read More »Bio-Rad launches blood screening controls in Europe
January 2023—Bio-Rad Laboratories has announced that Exact Diagnostics HBV, HCV, and HIV-1 screen controls are available in Europe. The controls are designed to monitor the performance of blood donor screening assays and are calibrated against the Third WHO International Standard for HBV (NIBSC code 10/264) and HIV-1 (10/152) and the Fourth WHO International Standard for HCV (06/102). The products are made of whole viruses in a citrate plasma matrix to ensure lot-to-lot reproducibility, have an 18-month shelf life from date of manufacture when stored at −20°C or below, and are stable for 24 hours when stored at 2° to 8°C.
Read More »Bio-Rad, NuProbe to develop digital PCR assays
December 2022—Bio-Rad Laboratories and NuProbe USA have entered into an agreement in which NuProbe will exclusively license its allele enrichment technologies to Bio-Rad for the development of multiplexed digital PCR assays. “Bio-Rad provides the leading solution for digital PCR, and we are committed to providing oncology researchers with technologies that enable everything from biomarker discovery to clinical trials and patient monitoring of minimal residual disease,” Simon May, EVP and president of Life Sciences at Bio-Rad, said in a joint press release. “We look forward to working with NuProbe USA to develop the next generation of highly multiplexed digital PCR assays as part of our expanding oncology offering.”
Read More »Bio-Rad introduces Unity Next Peer QC
December 2022—Bio-Rad Laboratories has launched Unity Next Peer QC, a quality control peer comparison software that provides access to peer reporting and comprehensive quality control data.
Read More »Bio-Rad introduces InteliQ QC for Beckman AU series analyzers
November 2022—Bio-Rad Laboratories has collaborated with Beckman Coulter to offer InteliQ load-and-go quality controls for use on Beckman Coulter DxC AU and AU series clinical chemistry analyzers.
Read More »Bio-Rad extends range of StarBright dyes
October 2022—Bio-Rad extended its range of StarBright dyes with the StarBright Violet 760, StarBright UltraViolet 575, and StarBright UltraViolet 605 dyes, to provide greater flexibility in multicolor flow cytometry panels. The company says the dyes offer improved brightness with narrow excitation and emission profiles for precise resolution, are resistant to photobleaching, and do not lose signal in fixation. They are compatible with the Bio-Rad ZE5 cell analyzer and S3e cell sorter and most flow cytometers without the need for special buffers.
Read More »Bio-Rad launches RNA library prep kit
September 2022—Bio-Rad Laboratories has launched its SEQuoia Express Stranded RNA Library Prep Kit. The three-tube kit uses a novel reverse transcriptase with ligation-free adapter addition chemistry to yield a reproducible, quantitative RNA sequencing library in three hours. The kit enables users to construct robust libraries that capture mRNA and long noncoding RNA (>200 bp) transcripts for differential gene expression analysis and novel transcript discovery.
Read More »Bio-Rad introduces quality control workbook series
September 2022—Bio-Rad Laboratories has launched the first workbook in a series that offers Professional Acknowledgement for Continuing Education credits.
Read More »Bio-Rad launches two negative run controls
August 2022—Bio-Rad Laboratories launched two new Exact Diagnostics products. The Exact Diagnostics Synthetic Negative Run Control is screened negative for nine targets, Anaplasma phagocytophilum, Babesia microti, Bartonella quintana, Borrelia burgdorferi, Ehrlichia chaffeensis, enterovirus coxsackievirus A9, HSV-1 and HSV-2, and varicella zoster virus. The Exact Diagnostics HAI Negative Run Control is screened negative for Clostridium difficile, methicillin-resistant Staphylococcus aureus, and methicillin-susceptible Staphylococcus aureus.
Read More »CFX Duet Real-Time PCR system
August 2022—Bio-Rad Laboratories has launched the CFX Duet Real-Time PCR system to support researchers in developing singleplex and duplex quantitative PCR assays. The CFX Duet system offers the robust thermal performance and proprietary, accurate optical shuttle system of the company’s CFX Opus system. It is a two-color system that is factory calibrated for common dyes and allows the quantification of up to two targets in genotyping and multiple gene expression analyses without the need for passive reference dyes. An additional fluorescence resonance energy transfer mode supports protein melt analysis for basic protein characterization.
Read More »Bio-Rad introduces anti-cemiplimab antibodies
June 2022—Bio-Rad Laboratories introduced a range of antibodies specific to cemiplimab (Libtayo) that inhibit the binding of the drug to its target, human programmed death receptor-1. The ready-made antibodies are suitable for developing selective and sensitive assays for the bioanalysis and drug monitoring of cemiplimab.
Read More »Bio-Rad SARS-CoV-2 variant neutralization antibody assays
February 2022—Bio-Rad Laboratories launched the Bio-Plex Pro Human SARS-CoV-2 Variant Neutralization Antibody Assays, for research use only. The assays allow scientists to measure neutralizing antibodies against wild-type and significant variants of the SARS-CoV-2 virus. The Bio-Plex Pro Human SARS-CoV-2 Variant Neutralization Antibody 11-Plex Panel is a ready-to-use 96-well kit containing premixed magnetic capture beads, a detection ACE2 receptor, a standard and positive control, and buffers that measure levels of neutralizing antibodies and percentage inhibition of the ACE2 receptor binding the viral antigens coupled on the beads.
Read More »Bio-Plex Pro IgA, IgM SARS-CoV-2 panels
December 2021—Bio-Rad Laboratories launched its Bio-Plex Pro Human IgA and IgM SARS-CoV-2 panels to detect IgA and IgM antibodies against four SARS-CoV-2 antigens. The two new panels along with the company’s existing Bio-Plex Pro Human IgG SARS-CoV-2 N/RBD/S1/S2 4-Plex panel aim to assist researchers in developing vaccines and to help public health researchers who perform seroprevalence studies based on serology specimens identify individuals who may have been exposed to SARS-CoV-2.
Read More »Bio-Rad launches CFX Opus Dx RT-PCR detection systems
Oct. 27, 2021—Bio-Rad Laboratories launched its CFX Opus 96 Dx and CFX Opus 384 Dx systems, real-time PCR detection systems that have been listed with the FDA for IVD testing and that meet the CE-IVD requirement for IVD use in Europe.
Read More »Bio-Rad adds SARS-CoV-2 to RP positive run control
Oct. 11, 2021—Bio-Rad announced its Exact Diagnostics RP Positive Run Control for syndromic respiratory panels is now available with inactivated whole virus for SARS-CoV-2.
Read More »Bio-Rad launches SARS-CoV-2/flu A/B kit in Europe
September 2021—Bio-Rad launched its Reliance SARS-CoV-2/FluA/FluB RT-PCR Kit (IVD) for European markets after having met the CE-IVD mark requirements.
Read More »Bio-Rad SARS-CoV-2 variant RT-PCR assays
July 2021—Bio-Rad Laboratories launched SARS-CoV-2 variant RT-PCR assays for research use only. The assays can detect SARS-CoV-2 variants of concern, including P.1, B.1.351, and B.1.1.7, by distinguishing specific mutations in SARS-CoV-2 using reverse transcription PCR, often prior to a next-generation–sequencing workflow for confirmation.
Read More »Bio-Plex IgG SARS-CoV-2 serology assays
June 2021—Bio-Rad Laboratories launched the Bio-Plex Pro Human IgG SARS-CoV-2 N/RBD/S1/S2 4-Plex Panel, a qualitative multiplex immunoassay kit that detects IgG antibodies against four SARS-CoV-2 antigens in less than three hours.
Read More »Bio-Rad Reliance RT-PCR COVID assays granted EUA
April 2021—Bio-Rad Laboratories announced that its Reliance SARS-CoV-2/FluA/FluB RT-PCR and Reliance SARS-CoV-2 RT-PCR assay kits were granted emergency use authorization by the FDA.
Read More »Bio-Rad enhances InteliQ QC line
April 2021—Bio-Rad Laboratories has enhanced its InteliQ liquid quality controls product line. InteliQ products have improved open-vial stability, and they are now available on Abbott Alinity and Roche Cobas systems.
Read More »Bio-Rad unveils online resource for immunoblotting
February 2021—Bio-Rad Laboratories launched an online library called Bio-Rad Western Blotting Learning Center. The center was created to provide researchers an online platform to learn about western blotting and offers information on the science of western blotting, best practices, tips and techniques, as well as guidance on how to troubleshoot experiments. It is available via the company’s website at bio-rad.com/LearnWestern.
Read More »Bio-Rad SARS-CoV-2, flu, RSV run controls
January 2021—Bio-Rad Laboratories launched its SARS-CoV-2, Flu, RSV positive and negative run controls. The products enable laboratories to evaluate day-to-day and lot-to-lot variation of their multiplexed molecular assay and test for operator proficiency.
Read More »Bio-Rad controls for COVID-19 testing
November 2020—Bio-Rad Laboratories launched its in vitro diagnostics Virotrol SARS-CoV-2 and Viroclear SARS-CoV-2 positive and negative quality controls for use in antibody testing of SARS-CoV-2. The serological controls are available for in vitro assay procedures in the U.S. and are CE marked for IVD markets outside the U.S.
Read More »RT-PCR system available for COVID-19 testing
July 2020—Bio-Rad Laboratories announced that its CFX96 Dx Real-Time PCR System has been listed with the FDA for IVD testing and may be used to help in the diagnosis of COVID-19.
Read More »Bio-Rad SARS-CoV-2 serology, ddPCR tests
June 2020—Bio-Rad Laboratories announced it was granted FDA emergency use authorization for the company’s SARS-CoV-2 Total Ab test. The test has also met the CE mark requirements for Europe.
Read More »Bio-Rad SARS-CoV-2 serology, ddPCR tests granted EUAs
May 6, 2020—Bio-Rad Laboratories announced it was granted FDA emergency use authorization for the company's SARS-CoV-2 Total Ab test, a blood-based immunoassay that
Read More »Bio-Rad launches SARS-CoV-2 standard
March 13, 2020—Bio-Rad announced it has launched, under its Exact Diagnostics product line, a SARS-CoV-2 standard to support laboratory assay validation of COVID-19 testing.
Read More »EDX respiratory panel control
March 2, 2020—Exact Diagnostics, which Bio-Rad acquired in 2019, launched EDX RP Positive Run Control, an unassayed external quality control intended to monitor the performance of clinical respiratory assays.
Read More »Bio-Rad ddPCR microsatelIite instability assay
July 10, 2019–Bio-Rad’s droplet digital PCR microsatellite instability research use only assay is available for early access customers.
Read More »Bio-Rad quality control for urinalysis testing
 April 2019—Bio-Rad Laboratories announced the launch of Quantify Advance Control, an independent quality control used to monitor the precision of laboratory urinalysis test procedures. The control contains human urine solution and offers 31 days of open vial stability for all analytes, including ketones, at room temperature.
April 2019—Bio-Rad Laboratories announced the launch of Quantify Advance Control, an independent quality control used to monitor the precision of laboratory urinalysis test procedures. The control contains human urine solution and offers 31 days of open vial stability for all analytes, including ketones, at room temperature.
Bio-Rad introduces Liquichek Serum Indices
March 2019—Bio-Rad Laboratories announced the availability of Liquichek Serum Indices, used as part of laboratory interference testing to monitor an instrument’s ability to detect hemolysis, icterus, and lipemia specimen interferences in patient samples.
Read More »Bio-Rad quality control
January 2019—Bio-Rad has announced its InteliQ selection of quality controls, available in barcoded, load-and-go tubes. This new configuration is designed to help optimize workflow efficiency and improve the laboratory’s risk management program.
Read More »Recombinant beta-2 microglobulin
October 2018—Bio-Rad announced the launch of recombinant beta-2 microglobulin from the company’s line of critical raw materials. This recombinant sourced tumor marker protein offers consistency and security over materials harvested from human sources.
Read More »FDA-cleared BioPlex assay, 10/17
October 2017—Bio-Rad Laboratories announced FDA clearance for its BioPlex 2200 ToRC IgM Assay, a fully automated assay for the detection of IgM class antibodies to Toxoplasma gondii, rubella, and cytomegalovirus offered in a multiplexed panel.
Read More »Bio-Rad quality controls, 9/17
September 2017—Bio-Rad Laboratories has added four analytes to the company’s Liquichek Tumor Marker Control. The updated control received FDA 510(k) clearance and the CE mark and is available for the immunoassay-based tumor marker testing market.
Read More »Genomic sequencing solutions, 3/17
March 2017—Illumina unveiled the NovaSeq series and announced the launch of the Illumina Bio-Rad Single-Cell Sequencing Solution at the J.P. Morgan Healthcare Conference in January.
Read More »Quality controls, critical raw materials, 10/16
October 2016—Bio-Rad Laboratories launched Amplichek II quality control, which was issued a de novo clearance from the FDA. Amplichek II is the first in a series of infectious disease controls that the company is introducing to the molecular diagnostic testing market. It is an independent, multianalyte quality control that monitors the performance of in vitro laboratory nucleic acid testing procedures for the qualitative detection of health care–associated infections.
Read More »Maternal serum control, 7/16
July 2016—Bio-Rad Laboratories released its Liquichek Maternal Serum II Control, a frozen liquid human serum-based material designed to monitor the precision of laboratory test procedures used during maternal serum second trimester screening.
Read More »CE IVD mark for digital PCR system, 5/16
May 2016—Bio-Rad Laboratories announced CE IVD marking for its QX200 Droplet Digital PCR System. The technology, which partitions a DNA or RNA sample into 20,000 droplets and amplifies targeted sequences within each droplet, allows scientists to precisely detect and quantify low concentrations of target DNA and RNA sequences.
Read More »Bio-Rad, Illumina partnership, 3/16
March 2016—Bio-Rad Laboratories and Illumina announced an exclusive partnership to develop a comprehensive next-generation sequencing workflow for single-cell analysis. The end-to-end commercial solution will enable high-throughput sequencing of thousands of individual cells.
Read More »FDA clearance for A1c testing system, 3/16
March 2016—Bio-Rad Laboratories announced that its D-100 System for A1c testing has obtained FDA clearance to monitor the long-term blood glucose control of individuals with diabetes, as an aid in the diagnosis of diabetes, and to help identify those who may be at risk for developing the disease.
Read More »Allergen controls, 10/15
Bio-Rad Laboratories released human-serum–based Lyphochek Allergen sIgE quality controls to monitor the precision of in vitro allergy test procedures.
Read More »Bio-Rad, Beckman Coulter exclusive distribution rights, 6/15
June 2015—Bio-Rad is extending its agreement with Beckman Coulter and has named the company exclusive global distributor of Bio-Rad’s Access HIV combo assay and the Access hepatitis C virus assay in select geographies (the products are not available in the U.S. or Vietnam).
Read More »Bio-Rad extends availability of vitamin D kit, 5/15
May 2015—Bio-Rad announced the launch of the BioPlex 2200 25-OH Vitamin D kit for use on the company’s BioPlex 2200 system in U.S. markets. The assay offers a fully automated method for the quantitative measurement of total 25-hydroxyvitamin D in human serum.
Read More »HIV-1/2 supplemental assay, 2/15
Bio-Rad Laboratories’ Geenius HIV-1/HIV-2 Supplemental Assay, which received premarket application approval from the FDA, can differentiate circulating antibodies to HIV types 1 and 2 in whole blood, serum, and plasma. The assay offers a three-step procedure that produces onscreen results in 30 minutes with full traceability.
Read More »Bio-Rad extends contract, 8/14
August 2014—Bio-Rad Laboratories has extended its contract with Premier, a U.S.-based group purchasing organization. The agreement includes Premier general level and ASCEND members.
Read More »Multianalyte quality controls, 4/14
April 2014—Bio-Rad Laboratories has a comprehensive line of multianalyte quality controls now available in Siemens Dimension Vista vials for use on Siemens Dimension Vista Intelligent Lab Systems.
Read More »Bio-Rad to acquire MorphoSys’ AbD Serotec, 2/13:113
MorphoSys in December signed a definitive agreement to sell its AbD Serotec research and diagnostic antibody segment to Bio-Rad Laboratories.
Read More » CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management