Editor: Frederick L. Kiechle, MD, PhD
Submit your pathology-related question for reply by appropriate medical consultants. CAP TODAY will make every effort to answer all relevant questions. However, those questions that are not of general interest may not receive a reply. For your question to be considered, you must include your name and address; this information will be omitted if your question is published in CAP TODAY.
Submit a QuestionQ. What is the total allowable error for lupus anticoagulant testing?
A.October 2023—Total allowable error is the maximum amount of error, combining bias and imprecision, that is deemed acceptable for an assay. It can be defined by regulatory agencies or calculated as 0.25 × (CVi2 + CVg2)0.5 + z × 0.5 × CVi, where CVi means within-subject coefficient of variation, CVg means between-subject coefficient of variation, and z represents the z-score of a desired confidence limit (for example, z = 1.65 for a one-sided 95 percent confidence interval).Published total allowable error (%) for common coagulation analytes are as follows:
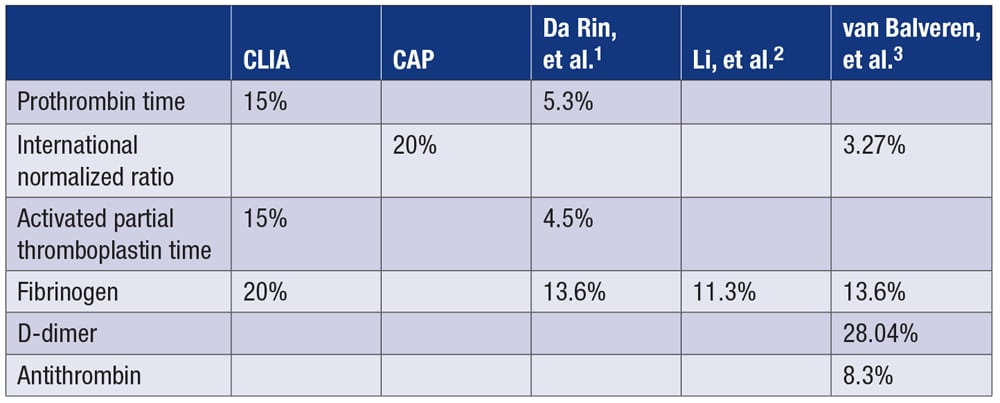
The European Federation of Clinical Chemistry and Laboratory Medicine and Westgard QC do not include lupus anticoagulants in their analyte variation databases.4,5 Despite updating its guidelines for lupus anticoagulant detection and interpretation, the International Society on Thrombosis and Haemostasis scientific and standardization committee’s subcommittee on lupus anticoagulant/antiphospholipid antibodies does not provide total allowable error recommendations for lupus anticoagulant.6
To our knowledge, only one article, by Shou, et al., has reported the within-subject and between-subject coefficients of variation of lupus anticoagulant, but it was specifically for dilute Russell viper venom time (DRVVT) using an Instrumentation Laboratory device.7 Based on those authors’ data, total allowable error can be calculated as follows:

Once the total allowable error for an analyte is defined, it can be used as a benchmark for gauging a test’s performance quantified by its total analytical error. The total analytical error for an assay can be calculated by combining the estimate of bias from a method-comparison study and the estimate of imprecision from a replication study: total analytical error = bias + 2 × standard deviations.8,9
- Da Rin G, Lippi G. Total laboratory automation of routine hemostasis testing. J Lab Autom. 2014;19(4):419–422.
- Li C, Sun Z, Liu Y, Zhou W, Wang Y, Peng M. Comparison among different measurement systems for fibrinogen using fresh samples and frozen samples. Clin Chim Acta. 2020;509:258–263.
- van Balveren JA, Huijskens MJ, Gemen EF, Péquériaux NC, Kusters R. Effects of time and temperature on 48 routine chemistry, haematology and coagulation analytes in whole blood samples. Ann Clin Biochem. 2017;54(4):448–462.
- Aarsand AK, Fernandez-Calle P, Webster C, et al. The EFLM biological variation database. European Federation of Clinical Chemistry and Laboratory Medicine. https://biologicalvariation.eu/
- Desirable biological variation database specifications. Westgard QC. www.westgard.com/biodatabase1.htm
- Devreese KMJ, de Groot PG, de Laat B, et al. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis: update of the guidelines for lupus anticoagulant detection and interpretation. J Thromb Haemost. 2020;18(11):2828–2839.
- Shou W, Chen Q, Wu W, Cui W. Biological variations of lupus anticoagulant, antithrombin, protein C, protein S, and von Willebrand factor assays. Semin Thromb Hemost. 2016;42(1):87–92.
- Westgard JO, Westgard SA. The quality of laboratory testing today: an assessment of sigma metrics for analytic quality using performance data from proficiency testing surveys and the CLIA criteria for acceptable performance. Am J Clin Pathol. 2006;125(3):343–354.
- Westgard JO, Westgard SA. Total analytical error: from concept to application. Clinical Laboratory News. Sept. 1, 2013. www.aacc.org/cln/articles/2013/september/total-analytic-error
Geoffrey Wool, MD, PhD
Associate Professor
Department of Pathology
Medical Director, Section of Transfusion, Hemostasis, and Apheresis Medicine
Director, Transfusion Medicine
Fellowship Program
University of Chicago
Chicago, Ill.
Member, CAP Hemostasis and Thrombosis Committee
Clarence Chan, MD, PhD
Chief Resident, Clinical Pathology
Department of Pathology
University of Chicago
Chicago, Ill.
Junior Member, CAP Hemostasis and Thrombosis Committee
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
