Editor: Frederick L. Kiechle, MD, PhD
Submit your pathology-related question for reply by appropriate medical consultants. CAP TODAY will make every effort to answer all relevant questions. However, those questions that are not of general interest may not receive a reply. For your question to be considered, you must include your name and address; this information will be omitted if your question is published in CAP TODAY.
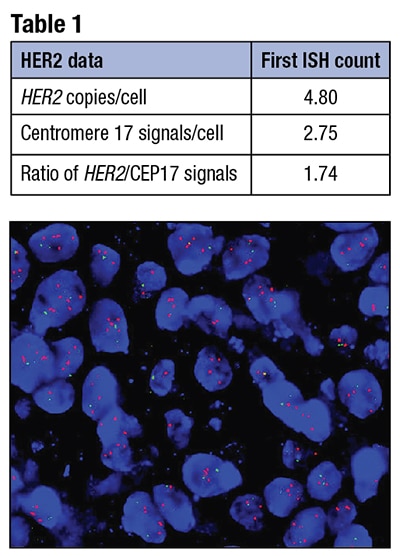
Fig. 1. Dual-probe in situ hybridization: HER2= red, CEP17= green, showing increased HER2 signals.
Q. I just read the 2018 HER2 guideline update. Can you provide an example of how a previously equivocal case is resolved under the new guideline?
A. March 2019—The 2018 revision to the American Society of Clinical Oncology/College of American Pathologists HER2 testing in breast cancer guideline contains several timely updates and a number of flow charts relevant to nonclassic in situ hybridization categories, along with interpretive and managerial comments that can be incorporated into pathology reporting.1 Here are examples:This ISH result would be in the equivocal category in the 2013 guideline, based on a HER2/CEP17 ratio of less than 2.0, in light of an average 4.8 HER2 copies per cell.
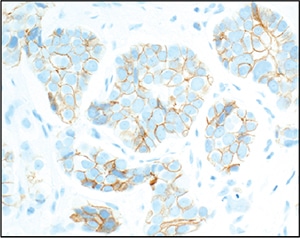
Fig. 2. Corresponding HER2 immunohistochemistry (4B5 clone), showing moderate intensity circumferential and noncircumferential staining, interpreted as 2+.
In figures 3 and 6 in the 2018 guideline, this is a “Group 4” result, requiring correlation with HER2 immunohistochemistry.1 HER2 IHC should be performed on the same block or sections used for ISH, preferably by the same institution, and interpreted in parallel (shown in Fig. 2 for this case). Alternate chromosome 17 probes are no longer necessary and are discouraged.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
