CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from the University of Washington, Seattle. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Shiva Khoobyari, MD
Eric Q. Konnick, MD, MS
 October 2018—Mosaic RASopathies result from postzygotic (“mosaic”) mutations in RAS-family genes.1 These disorders are characterized by dysregulation of RAS signaling pathways, resulting in overgrowth of affected tissues, and an increased risk of secondary transformation. Sensitive molecular diagnostic methods have been used to identify activating mutations in affected tissues and unravel the pathogenesis of the disorders.1-3 Nevus sebaceus and Schimmelpenning syndrome are mosaic RASopathies with an elevated risk of malignant transformation,1 and their accurate diagnosis has significant prognostic and therapeutic implications.4,5 Here, we discuss a clinical case in which genomic sequencing was applied to confirm the diagnosis of a potential Schimmelpenning/nevus sebaceus syndrome in a patient for whom there was a high clinical suspicion but an inconclusive microscopic assessment of grossly affected tissue.
October 2018—Mosaic RASopathies result from postzygotic (“mosaic”) mutations in RAS-family genes.1 These disorders are characterized by dysregulation of RAS signaling pathways, resulting in overgrowth of affected tissues, and an increased risk of secondary transformation. Sensitive molecular diagnostic methods have been used to identify activating mutations in affected tissues and unravel the pathogenesis of the disorders.1-3 Nevus sebaceus and Schimmelpenning syndrome are mosaic RASopathies with an elevated risk of malignant transformation,1 and their accurate diagnosis has significant prognostic and therapeutic implications.4,5 Here, we discuss a clinical case in which genomic sequencing was applied to confirm the diagnosis of a potential Schimmelpenning/nevus sebaceus syndrome in a patient for whom there was a high clinical suspicion but an inconclusive microscopic assessment of grossly affected tissue.
Case. A five-year-old African-American boy presented with a ~3.5 cm × ~2.0 cm tan papillomatous plaque on the left parietal scalp. His history was significant for cognitive developmental delay and epileptic seizures, with Fragile X syndrome testing showing one unexpanded FMR1 allele. Histologic examination of a 4-mm punch biopsy showed only a few primitive hair follicles and small sebaceous lobules (Fig. 1). Although the microscopic findings were nonspecific, the histologic differential diagnosis included skin with no diagnostic alteration, prepubertal stage of a nevus sebaceus, or other somatic overgrowth syndromes. Targeted next-generation sequencing using a clinically validated method6 (see “Methods”) was performed on 2.8 µg DNA extracted from a formalin-fixed, paraffin-embedded skin sample. Average sequencing coverage was 865-fold, and only an activating HRAS p.Gly13Arg mutation (NM_005343.2:c.37G>C) was identified. This mutation was detected in only 20 percent of sequencing reads (27 of 134 reads), supporting its presence in only a subset of the tested cells, consistent with somatic mosaicism. No mutations were detected in KRAS, NRAS, PTEN, or PIK3CA, which have also been described in similar lesions.
Discussion. Nevus sebaceus (NS) is a common congenital skin hamartoma, with the clinical appearance characterized by yellow, waxy, hairless plaques on the scalp, face, or neck.1 NS shows progressive growth and becomes thickened and verrucous as the patient matures.7 NS is typically a solitary lesion with no other clinical association. However, in approximately seven percent of cases, it is the hallmark lesion of Schimmelpenning syndrome (MIM: 163200),8 a multiorgan disorder characterized by the association of nevus sebaceus with extra-cutaneous abnormalities, including ocular, skeletal, cardiovascular, and central nervous system anomalies, including seizures. Sebaceous nevi develop along Blaschko’s lines, characterized by S- or V-shaped strips of affected skin.9 This clinical presentation suggests that the underlying etiology may be due to genetic mosaicism.10
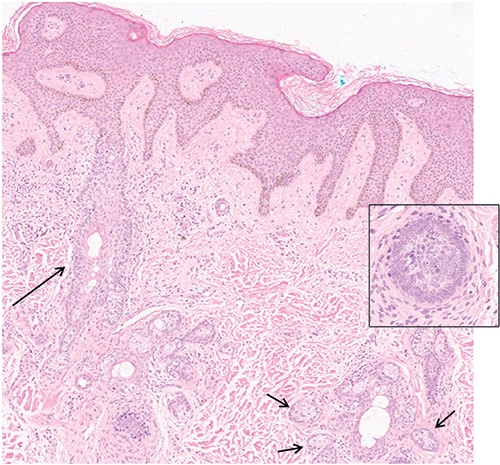
Fig. 1. Photomicrograph of the lesion shows dilated follicular infundibula (long arrow) with few attached budding undifferentiated hair follicles. There are small sebaceous glands (short arrows) next to dilated sebaceous ducts (hematoxylin-eosin stain; × 200). lnset: undifferentiated hair follicles (hematoxylin-eosin stain; × 400).
The phenotype of mosaic disorders is determined by when the mutation arises in development, and the clinical presentations are accordingly varied. In NS, it has been proposed that the genetic mosaicism is limited to the skin, while the mutation is observed in other organs in Schimmelpenning syndrome.10 Other conditions, such as hamartoma tumor syndrome and PIK3CA-related overgrowth syndrome, involve postzygotic mutations in PTEN and PIK3CA, respectively. These syndromes also can present with skin lesions in a patchy distribution, which raise suspicion for genetic mosaicism.11
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
