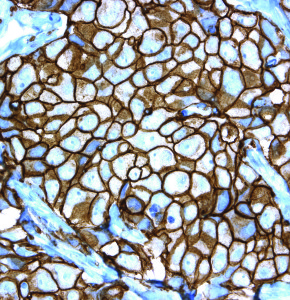
 February 2014—Ventana Medical Systems’ HER2/neu (4B5) Rabbit Monoclonal Primary Antibody assay is a companion diagnostic for detecting HER2 protein expression in patients who, in countries where the drugs are approved, may be appropriate candidates for Perjeta (pertuzumab) and Kadcyla (ado-trastuzumab emtansine).
February 2014—Ventana Medical Systems’ HER2/neu (4B5) Rabbit Monoclonal Primary Antibody assay is a companion diagnostic for detecting HER2 protein expression in patients who, in countries where the drugs are approved, may be appropriate candidates for Perjeta (pertuzumab) and Kadcyla (ado-trastuzumab emtansine).
Previously, the Ventana HER2 (4B5) test was labeled only for the identification of HER2-positive breast and gastric cancer for which Herceptin (trastuzumab) treatment was being considered.
Ventana, a member of the Roche Group, has worked not only with Roche but with more than 45 other biopharmaceutical partners over the past decade and is engaged now in more than 150 collaborative projects to develop and commercialize companion diagnostics worldwide.
The Ventana HER2 (4B5) IHC assay is a CE-IVD companion diagnostic available outside the United States.
Ventana Medical Systems, 520-877-7288
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
