Karen Lusky
July 2017—Kisha Mitchell Richards, MBBS, once took a picture of the ocean as she went around a bend in the road traveling from Negril to Montego Bay in Jamaica. She showed that photo in the second half of a CAP16 session to prepare the audience to shift gears, as she put it, from the first speaker’s talk on medical liver disease (see “Liver injury patterns: pitfalls and pointers,” March 2017) to hers on hepatic neoplasms. “So for me, we are about to go around a bend to things of sheer beauty,” she said, referring to immunohistochemistry stains in the neoplastic liver. “Unfortunately, that which is beautiful to the pathologist is not often great for the patient. That’s our usual practice,” said Dr. Richards, a pathologist at Greenwich Hospital, Yale New Haven Health, Greenwich, Conn.
“We are going to look at the H&E morphology because at the end of the day, our bread and butter is H&E,” she told attendees. “The immunohistochemistry is beautiful, lovely to see, often helpful, but sometimes confounding,” she said, noting that she planned to talk about some of those issues and suggest when to use IHC and which stains to use.
Dr. Richards also said that it’s important to recognize the spectrum of hepatocellular neoplasms. “Now it’s not that we have all these cutting-edge treatments for hepatocellular carcinoma. It’s one of those that’s kind of the laggard in terms of treatment,” in part, she says, because there’s not as much tissue available to study. “Everybody gets a diagnosis of breast cancer from tissue. People get radiologic diagnoses of HCC and they never have tissue. They only come to us when it’s failed.” She predicts that’s going to change.
Dr. Richards talked about hepatocellular carcinomas, hepatoid tumors, the hepatocellular-cholangiocarcinoma mixed tumor, and benign neoplasms. She presented three main case studies. The focus of the first was the well-differentiated lesion.
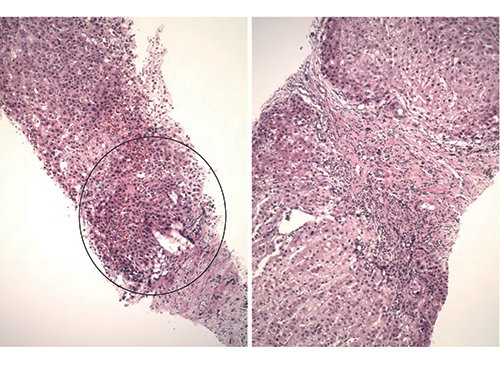
Fig. 1
A 32-year-old woman seen for abdominal pain had multiple liver masses. “Additional evaluation identified congenital absence of the portal vein with drainage of the portal splenic confluence directly into the IVC [inferior vena cava],” she said. “So she had a vascular abnormality that she’d had since birth.” The woman underwent a partial hepatectomy after a biopsy of one of the largest liver masses appeared atypical.
Dr. Richards showed an image of the initial atypical biopsy, pointing out fibrous bands on the right side (Fig. 1). “This is not a patient who is known to be cirrhotic but you have these thick fibrous septa and one would start to think of an FNH [focal nodular hyperplasia], particularly in someone with a vascular abnormality,” she said. “But some things that were kind of concerning to the person looking at this is that you start to get the sense that there is a little bit of increase in cell density like there are too many nuclei in a portion of the biopsy” (Fig. 1).
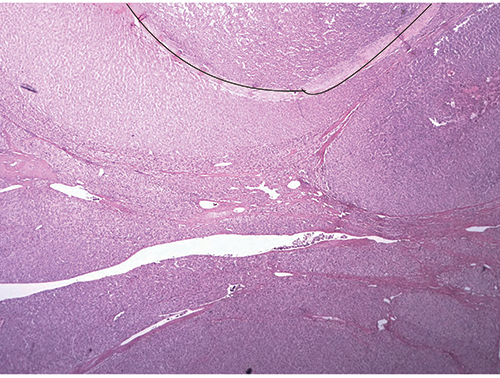
Fig. 2
Displaying the image of one area from the partial hepatectomy (Fig. 2), Dr. Richards said “You can still see the persistence of some of these areas with these thick fibrous septa coursing through the liver and these vague areas of nodularity that certainly look different under high power. Even though you have the fibrous septa, some areas appear somewhat bland, although the cells are a bit small and look somewhat adenoma-like; there are no portal tracts. You have fibrous septa but no portal tracts, and that’s the beginning of the feature that this is neoplastic: the absence of portal tracts.
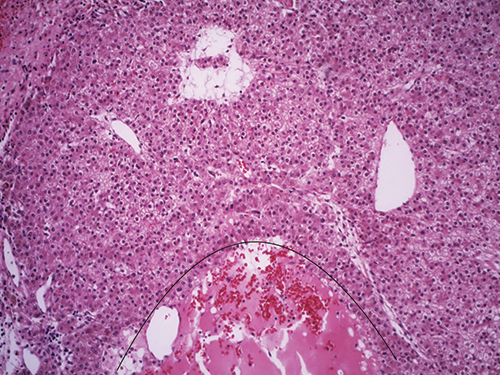
Fig. 3
“Then you also see, which we commonly see in adenomas, there’s almost this peliotic-like appearance [and] again complete absence of portal tracts and no bile ducts are seen (Fig. 3). And the hepatocytes in between this suggest something a bit more sinister. What is going on? Some of the cells look a little bit small when you compare them.”
Dr. Richards pointed to another area (Fig. 4) that had more than a twofold increase in cell density. “If you took the little area and just drew a box around it, normal liver in this focus should not have so many nuclei in that small area,” she said. “So certainly you have greater than a twofold increase in cell density in this lesion that has a heterogeneous appearance.
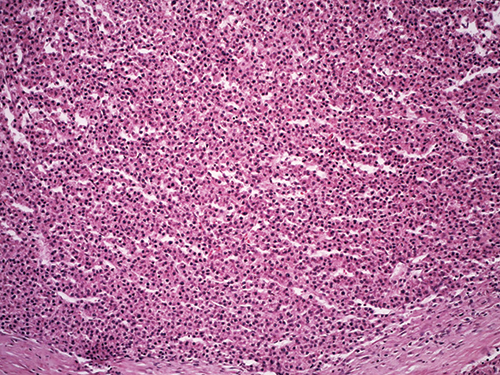
Fig. 4
“And then in the background of the liver, she had typical features of FNH—fibrous septa with ductular proliferation and otherwise bland and unremarkable hepatocytes. And the diagnosis was made (Fig. 5) that this patient had a separate FNH and then a well-differentiated hepatocellular carcinoma arising in an adenoma.” Dr. Richards said the case demonstrates something she used to tell residents, and actually picked up from a resident: “You can have fleas and lice.”
As for FNH, ”the consensus theory,” Dr. Richards tells CAP TODAY, “is that it’s not even a neoplasm. It’s more a reactive process to changes in blood flow. For example, FNHs may occur in patients who have congenital absence of their portal vein. With the variability of blood flow throughout the liver, FNH occurs in that setting. But that same predisposition to FNH, that abnormal blood flow, can also predispose patients to adenomas and HCC.” By contrast, in the cirrhotic patient, the risk of developing HCC has more to do with the ongoing inflammation.
With new developments and new molecular tools, she noted, “we do know that in theory you can get FNH and adenomas in cirrhotic livers.” However, “It’s very difficult to make that diagnosis especially on biopsy because the worry always in cirrhosis is that what you have developing is a dysplastic nodule and then carcinoma.”
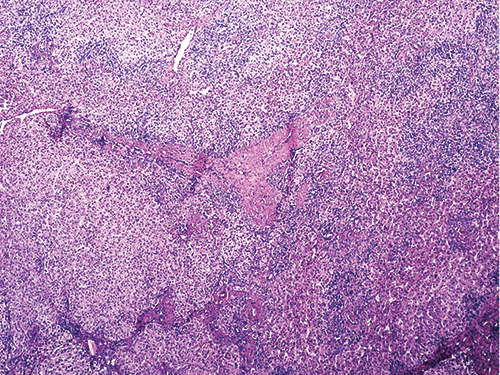
Fig. 5
In the cirrhotic liver, “we think there is really allegedly a nice orderly progression. First you get dysplastic foci and usually those are small cell changes,” Dr. Richards said. Next there are regenerative nodules, and once those acquire atypical features, they are called low-grade dysplastic nodules. “And when the features are a little bit more, we call those high grade, and then eventually you get either a small or a progressed HCC.”
She calls the progressed HCC the typical one that all can recognize—it meets all the criteria. The pathologist can see the thickened plates, the endothelialization of sinusoids. “But there is that small HCC that gives us a lot of problems. It can be an early HCC and sometimes it can have variable features.”
The HCC won’t necessarily have a lot of bile ducts but the pathologist may see varying numbers of unpaired arteries, Dr. Richards continued. “So sometimes you might see arteries with ducts; sometimes you might not. Some of these will have vague focal areas with pseudoglandular patterns; some of these will have increased cell density but they may not all have all of these features.” Interestingly, some studies show that some lesions of about or less than two centimeters tend to have diffuse fatty change, she added. Thus, if a cirrhotic liver is being evaluated and a small lesion is seen that has unpaired arteries and diffuse fatty change, “be careful about kind of excusing that as an adenoma or something else or just fatty change,” especially if the background liver doesn’t have fatty change. That lesion may be a well-differentiated HCC.
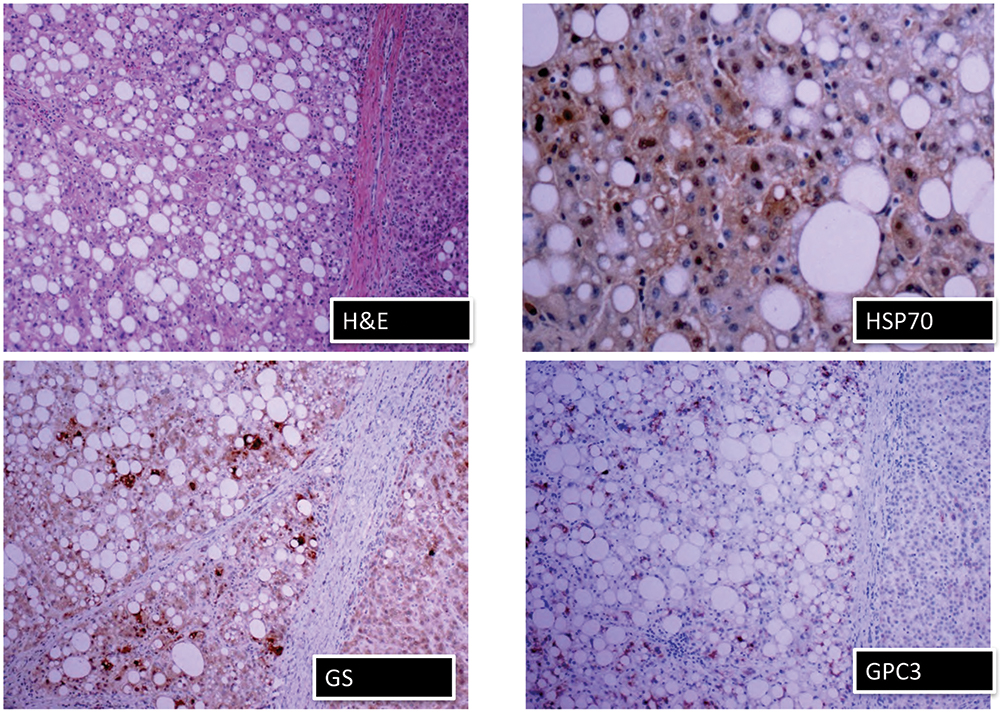
Fig. 6
As the lesion starts losing bile ducts and has unpaired arteries, it becomes hypervascular, which the radiologist sees as increased intensity in the arterial phase, she says. And then in the venous phase, the radiologist sees that lesion “wash out,” which is a radiologic feature of HCC. “That’s how they diagnose it based on the premise of hypervascularity.”
“All tumors don’t, however, read the book,” Dr. Richards said. Sometimes an HCC is hypervascular but doesn’t necessarily wash out, though it’s unclear why that may occur. “Or you may have a lesion that doesn’t really become hypervascular. Let’s say it doesn’t have a lot of unpaired arteries; it has some but not all and it retains a few portal tracts like in the early phase of development of HCC. So sometimes you can have an aberration and that’s when the radiologist can be wrong. They can say it’s benign when it’s not.”
Serum alpha-fetoprotein is important but only in some settings because many HCCs may not produce AFP, Dr. Richards says. “If there is no increase in AFP, it doesn’t necessarily mean it’s not malignant. It’s much more helpful if you do get a marked increase in AFP because HCC is the most common tumor that produces an increase in serum AFP.”
For HCC in the cirrhotic liver, it is recommended that an immunohistochemistry panel of glypican 3, glutamine synthetase, and HSP70 (heat shock protein 70) can be useful, Dr. Richards said. “And if any two of those three are positive, then that suggests hepatocellular carcinoma.”
She shared images of an H&E stain and the three immunostains in a well-differentiated HCC (Fig. 6). “In a perfect world, when you have that panel, this is what you would do,” she said. “Here is your H&E. You have glutamine synthetase. Even though this is steatotic, there is some positivity. But in those same areas, there’s glypican 3 positivity and there’s also HSP70, which can be very focal, and that’s why you need a high power there.” Pathologists may not have all three of those stains, she noted. “If you were going to get those stains,” she told attendees, “I’d say that if you had a budget and you could get glutamine synthetase and the glypican 3, your money is better spent there than investing in the HSP70 because that can be focal, it can be difficult to interpret, it can be difficult to use.”
Dr. Richards noted that HCC is the “great masquerader.” Classic HCC can be recognized quite easily, she says, but there are a number of lesion types that are also HCC that are not recognized very well.
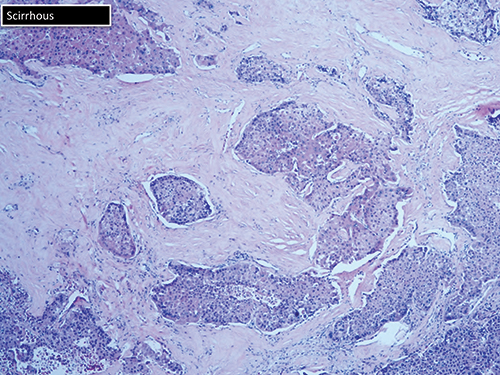
Fig. 7
To review the subtypes, she shared her “coloring book of HCC,” starting with an image of a classic HCC, which she observed has “beautiful thickened plates and you can highlight with reticulin or you may have reticulin loss.” (Reticulin is not an immunostain.)
Some patients have the steatotic variant. “About 13 percent to 15 percent of these are associated with
steatohepatitis and the metabolic syndrome,” she said. Dr. Richards cautioned that there may be a loss of reticulin in steatohepatitis without HCC.
The scirrhous variant can be mistaken for cholangiocarcinoma (Fig. 7). “Just by looking at it, they call it high-grade cholangio. And you may need to do your hepatocellular stains.” The markers of liver origin are HepPar1, arginase 1, polyclonal CEA, and glypican 3.
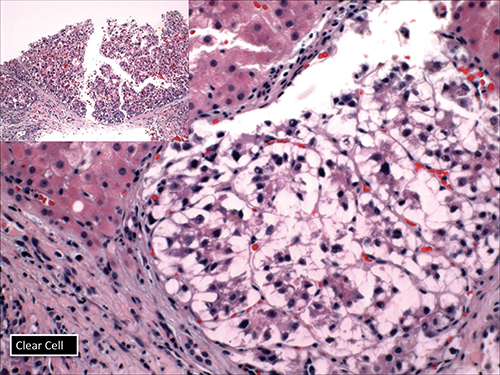
Fig. 8
Dr. Richards also displayed the clear cell variant of HCC (Fig. 8), which she said might be confused with metastatic renal cell cancer or something else. “But be aware that you can have clear cell features in many carcinomas, including cholangiocarcinoma.”
Then there is the fibrolamellar variant that has large cells typically with ovoid pale bodies and lamellar collagen, which may not be seen on the biopsy but the size of the cells should be recognized (Fig. 9). “And this does not happen in the setting of cirrhosis,” she said.
The lymphoepithelioma-like variant is not that common, she said. A recent paper reported that it’s “a little bit enriched for expression of PD-L1. So that’s something to keep in the back of your mind because you know once there’s a drug, they are going to want you to do the stain eventually.” She showed the image of an arginase stain (Fig. 10) that was positive in the hepatocytes, which she said confirmed the lesion was HCC rather than some other type of tumor that has lymphoid epithelial features.
The sarcomatoid variant usually appears quite spindly (Fig. 11). “One would look at this and just call this a high-grade malignant neoplasm if you don’t do your hepatocyte markers, which in this case an arginase is a very good stain,” she said, noting the strong positivity.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
