Anne Paxton
April 2018—If you were asked to pick a place on the map where problems with detecting a mutant strain of an infectious disease would likely come to light, the capital of Nebraska might not be your first guess. But in Lincoln, in 2017, a hemodialysis patient who was vaccinated against hepatitis B virus in 2010 and had repeatedly tested negative for HBV surface antigen was found to have had undiagnosed chronic hepatitis B. The case led state and local public health officials to investigate and find evidence that some hepatitis B surface antigen (HBsAg) assays were not picking up particular HBV mutant strains.
These false-negative HBV tests and their ramifications were the subject of a “Notes from the Field” in the Centers for Disease Control and Prevention’s March 16 Morbidity and Mortality Weekly Report, titled “False-negative hepatitis B surface antigen test results in a hemodialysis patient—Nebraska, 2017” (Hendrickson B, et al. 67[10]:311–312). The case highlights a unique challenge associated with detecting HBV infections when a surface antigen mutation is present, says Blake Hendrickson, MPH, vaccine-preventable disease epidemiologist for the Nebraska Department of Health and Human Services and lead author of the MMWR report.
But, he adds, “it was almost by accident that this was identified.”
“Typically, after someone receives the HBV vaccine series and tests negative, they’re not routinely tested for a surface antigen because it’s assumed they’re protected and won’t become infected,” Hendrickson says. “In 2010, this patient tested negative for HBsAg and positive for hepatitis B surface antibody. That indicated that the patient was not infected and the vaccine should be protective.”
HBsAg testing wasn’t done again until 2016. That year, when the patient was hospitalized for acute shortness of breath, a workup showed he was positive for hepatitis B, based on another surface antigen test. The hospital and the dialysis center used different labs, and the hospital’s test was positive while the dialysis center’s were repeatedly negative.
“Because of those discordant results and the uncertainty of the patient’s status, the doctor reached out to us. That’s an unusual situation—to have a patient tested at the same time at two different labs,” Hendrickson says. “But because of that, the discordant results were identified and could be investigated further. The CDC, which knew about this phenomenon, was willing to perform some additional testing for us.” It was also out of the ordinary that the doctor in this case took the initiative to contact public health authorities. “He felt it was intriguing and concerning enough to contact us, and we went through a process to figure out what was going on. But in most situations, there wouldn’t be a reason to follow up to this degree.”
The CDC estimates that about 1.2 million people in the U.S. are living with HBV, although there is concern about underreporting of chronic hepatitis B. Other studies have estimated this number to be as high as 2.2 million. That would still be a small percentage of the approximately 350 million HBV-infected people worldwide.
“Globally, greater than three percent carry HBV as a chronic infection, and this has been shown in different prevalence studies internationally to be a significant number of people,” he says. “The interventions that the public health and clinical community are using to reduce the prevalence of HBV include not only stopping bloodborne transmission but also preventing mother-to-child transmission during birth. This requires proper diagnosis of the mother’s HBsAg status prior to delivery and providing vaccination and HBV immunoglobulin to the infant. Ninety percent of infants who are perinatally infected with hepatitis B will develop chronic disease, so not detecting these persons accurately would be a huge limitation.”
The vast majority of people in the U.S. who are hepatitis B positive are from endemic countries and are of certain races and ethnicities, Hendrickson points out. “In Nebraska we don’t have nearly as much diversity or overall population size compared to other states, so this was a somewhat strange thing to find.” For the perinatal HBV vaccination program that the state conducts, only about 70 HBV-positive mothers were identified last year. “In larger states with many more chronic carriers, not detecting mutant hepatitis B viruses could become a larger-scale issue.”
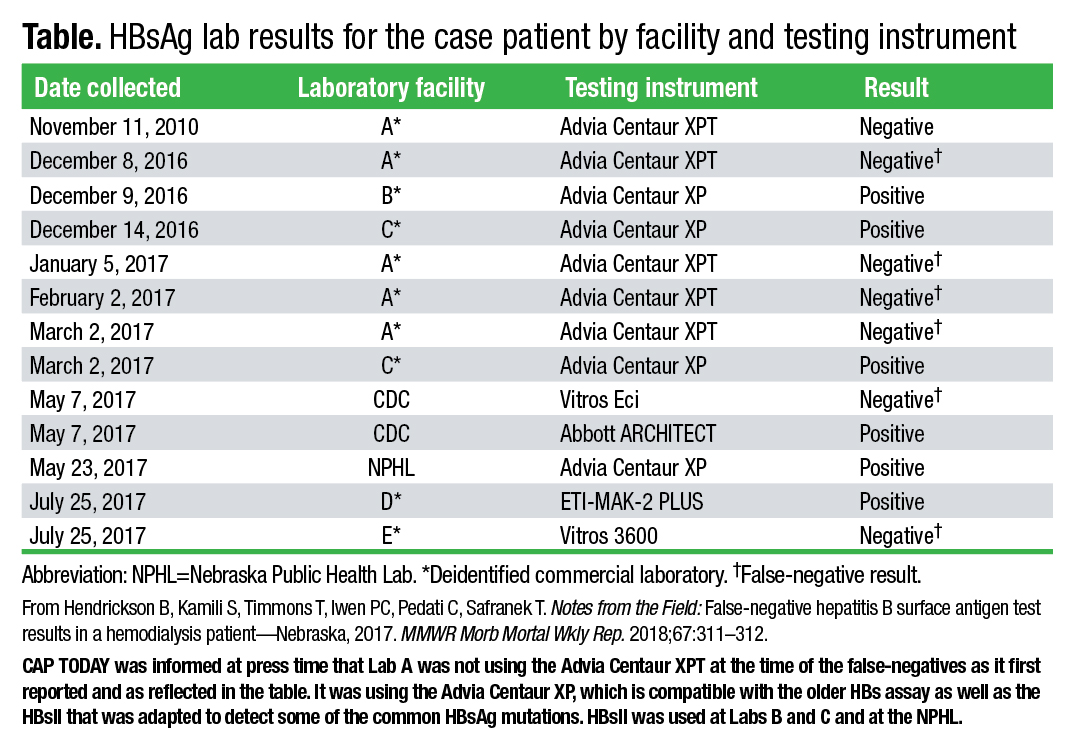
When they began looking into the false-negative case in Lincoln, the public health investigators decided to run additional tests and compare results. In total, 12 surface antigen tests were run on a blood sample from the patient in 2016 and 2017 by several commercial laboratories, a hospital, the CDC, and the Nebraska Public Health Laboratory. Some tests produced false-negative results—those on the Vitros 3600, Vitros Eci, and Advia Centaur XP with the older assay. The tests performed on the Abbott Architect, ETI-MAK-2 Plus, and on the Advia Centaur XP using the updated HBsAg assay had positive results (Table).
The CDC also found high HBV DNA levels in the patient’s blood sample and, by sequencing the S gene of HBV DNA, identified the sG145R surface antigen mutation, which is associated with false-negative results and explains the failure of multiple tests to identify the patient as HBsAg positive. Some studies, as the MMWR report notes, have estimated that sG145R and other mutant strains are found in six to 12 percent of chronic HBV carriers. “So the surface antigen genes were mutated in a way that some assays that hadn’t been updated wouldn’t catch it. But more recent assays were able to catch it,” Hendrickson says.
While the MMWR report deidentifies the commercial laboratories in which the patient’s testing was performed, Hendrickson and his coauthors from the CDC and Nebraska state and county public health agencies decided to identify the instruments and manufacturers. “It is concerning that this is one of the more common mutations for hepatitis B, and with these frequently used testing platforms not being able to detect it, that leads us to believe this is something of public health importance.”
Sensitivity and specificity studies relating to the specific surface antigen kits used in this case show that the test manufacturers prioritize sensitivity, Hendrickson says. “It’s better to have a false-positive than it is to have a false-negative, as in this scenario. There are a variety of hepatitis B testing indicators, and the surface antigen test is kind of the preemptive one. If that’s positive, maybe you should do a panel to confirm the diagnosis, check viral load, or even check for co-infections with other hepatitis viruses.”
The sensitivity of surface antigen tests is typically high. “But it would be reduced when you’re considering viruses that have a surface antigen mutation, compared to a wild type.”
The mutant strain of HBV in this case reflects the typical change in viruses over time. “It’s an example of genetic drift, and that’s affected by the vaccines and immunoglobulin interventions because of natural selection and the ability of the virus to naturally change because of a selective pressure.” However, Hendrickson adds, therapy and vaccines also prevent many infections and reduce the amount of hepatitis B dramatically. “So the overall burden is being reduced, but the complexity of the virus is increasing.”

Hendrickson
Hepatitis B happens to have a particularly wide genetic diversity, he says. HBV replicates by RNA, so it doesn’t have the same mechanism of correcting transcription errors as other viruses. “That actually leads to a higher mutation rate than what you would see in DNA-based viruses.” A further complexity of testing for hepatitis B with surface antigen tests is that not every hepatitis B virus in the body is going to be mutated, even if there is some level of mutant virus present. “The concentration of the mutant virus even within the individual being tested affects how likely a testing instrument is able to detect it. If the vast majority of virus in an individual is wild type, most testing platforms are going to be able to detect it. But there’s an unknown threshold where, as in this case for example, we had mainstream tests that completely missed it.”
When tests are approved for commercial availability, the frontline demonstration of effectiveness includes the ability to detect different viruses that might be prevalent. “You can’t just base that testing on your standard wild-type or known prevailing viruses. You need to include these mutant panels in that assessment of your test.”
The diagnostics manufacturers are addressing the need to test for more variants, Hendrickson believes. “The Abbott Architect is currently the gold standard for detecting HBsAg mutants and being up to date in knowing what mutants are out there and which should be incorporated. Siemens Advia Centaur was the culprit in this case, but I wouldn’t blame the manufacturer. They have also developed newer and improved kits to detect these mutants. The problem here is that the reference lab for the dialysis center wasn’t using the improved kits at that time.” The manufacturers encourage clients to modify and update their assays to the newest product, he adds. “But these are expensive products and there are many factors to consider beyond newly identified mutants.”
More sensitive tests such as PCR have advantages over surface antigen tests but are unlikely to be used as firstline screening tests, Hendrickson says. “PCR is able to produce a result that’s quantified into a viral load, which can be clinically useful in indicating the degree of infection and also the potential for transmission. But PCR testing is significantly more expensive.”
Siemens Healthineers’ Sai Patibandla, PhD, senior director of immunoassay development, laboratory diagnostics, says such approaches have not to her knowledge been recommended. “In the U.S., with such a low incidence rate of chronic hepatitis and an even lower rate of viral mutations, it could be a very burdensome cost to the health care system without adding much value.”
“What a lot of laboratories do is to test with a hepatitis panel,” Hendrickson says, “including HBV, plus A, C, and sometimes further testing for hepatitis D or E. So if a patient has jaundice or other indicators of hepatitis such as elevated liver enzymes, many times doctors will order the hepatitis panel to determine if symptoms are caused by viral hepatitis.” Core antibody testing can also be helpful. “A total core antibody shows if someone was ever infected in their entire life. One of the benefits is that someone vaccinated against hepatitis B is not going to develop the core antibody response, whereas if you have a surface antibody that tests positive, you can’t be sure whether that was caused by a vaccine or an actual infection. The vaccine causes an immunity response, so you have to take that into consideration when interpreting the tests.” The HBV vaccine, for instance, can cause a positive result, especially within the first month after vaccination. “But the vaccine does not cause a core antibody response.”
The total core antibody test is different from the IgM core antibody test, Hendrickson notes. “The IgM is very useful because it indicates a pretty recent infection, and that would be most relevant for an acute hepatitis B patient.” Many people who contract hepatitis B don’t show symptoms or have any problems for a long period of time, but HBV can also cause an acute infection, with jaundice and other classic hepatitis symptoms developing after infection.
Despite the number of tests of the Lincoln dialysis patient, “It’s still unclear how he became infected,” Hendrickson says. “We can’t tell if it was in the distant past or after he was vaccinated and first tested in 2010.”
After this patient’s sample was retested, the Nebraska health department conducted an epidemiologic investigation, screening the patient’s family and dialysis clinic contacts with an HBsAg assay that is sensitive to the sG145R mutation or by HBV DNA. They found no evidence of HBV transmission despite the contacts’ potential exposures to the patient. However, a survey of all 23 laboratories in the region that do surface antigen testing found that nine are using tests not known to detect the common surface antigen mutations. “So nine out of 23 labs that are testing Nebraskans most frequently are using tests that aren’t known to be sensitive to the mutations, which is adding to our thinking that this could be a public health concern,” Hendrickson points out.
Though it has been suggested that the CDC or the Infectious Diseases Society of America issue new guidelines on detecting mutant strains, Hendrickson is not sure such a step is needed. CDC recommendations for preventing transmission of infections among dialysis patients with chronic hepatitis B were made in 2001 and are updated periodically (the 2016 update is at www.cdc.gov/dialysis/guidelines/index.html). Although he sees the case reported in MMWR as an important limitation, the motivation for publishing the report was that it’s not well recognized that HBsAg tests may not pick up a mutation. “Getting this information out to the laboratory community is going to raise some level of awareness to it. It may not be something that necessarily warrants guideline changes.” A lot of the considerations that would be related to the value of the new test requirements lie with the FDA rather than the CDC, he adds.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
