
Michele A. Smith, MS, SCT(ASCP)
August 2015—To gear up for the change from ICD-9, the Centers for Medicare and Medicaid Services has provided updates and training and has kept ICD-9 changes to a minimum in an effort to build a strong crosswalk to ICD-10. Last year, the U.S. was given one more year to prepare, but that will not be the case this year. In fewer than 75 days, on Oct. 1, the U.S. will convert to ICD-10 coding.
Much of the heavy lifting of the ICD-10 transition takes place electronically, meaning that health information management personnel are doing most of the infrastructure work in any given health care department. However, it is important also for others to understand and be part of the testing to ensure an accurate transition.
Laboratories have always been a bit different in terms of coding and billing training and information, and this will remain the same for the ICD-10 implementation. But all of the training and information provided through the CMS can be applied to the laboratory. The key is for laboratories to be participants in all discussions and testing done for ICD-10 within their institutions. Let’s look at how the laboratory can use the various CMS training opportunities.
Roadmap. The CMS has provided a series of training opportunities or roadmaps on how to be ready for the ICD-10 implementation and discusses five steps to help in the transition: make a plan, train staff, update the processes, talk to outside partners (in this case for billing and payment purposes), and test the system and the process. The steps are much like what we do in the laboratory every day.
Hospitals are well along in taking these steps, but the question laboratory leaders need to ask is: Has the laboratory participated in the roadmap steps? If the answer is no, then the time is right to jump into the last months of testing to ensure that the lab’s reimbursements are not negatively affected on Oct. 1.
Planning. Here is how the lab fits into each of the five steps of the roadmap for planning and implementing the ICD-10 transition.
Make a plan. Obtain access to ICD-10 codes. These codes are now on the CMS website: www.cms.gov/Medicare/Coding/ICD10/2016-ICD-10-CM-and-GEMs.html. This is the first step in looking at the code changes from ICD-9 to ICD-10 in both PDF and XML coding formats. The goal is to be aware of the new codes and how they will be implemented in the lab information system, electronic health record, and billing formats.
The Pareto principle (80-20 rule) may be useful when deciding where to start. Assess your test menu and look first to those tests that provide the majority of your revenue. From these tests, generate a report that lists the ICD-9 codes used to support the medical necessity for those tests. From here, crosswalk the most often used ICD-9 codes into ICD-10.
Train your staff. In the laboratory, we often feel that the coding is up to the clinician and that we do not have much to do with it. I would argue that we do have more responsibility. First, we are bound to only provide testing on orders that are medically necessary. In this way, we need to not only review the test orders and codes but also understand what the codes mean so we can ensure efficient and accurate results. As laboratory professionals and diagnostic experts, we should be asking for clarification of orders and tests when they are not specific or when things don’t appear to be right.
For example, a test order for a BAL is received in the laboratory. The code of cough is much less specific than, say, a cough from a patient who is immunocompromised. While both provide a medical necessity reason for the procedure, the second tells a more complete story.
Some may say this information is easily found in the electronic record as documented in the visit. However, not all laboratories have full access to the EHR and not all LISs are able to “talk” electronically with other health information systems. In many labs, the conversation still takes place over the phone or via fax.
Update your processes. Several years ago the CMS updated its billing practices to become electronic. Most labs as well as other clinical providers moved to this format as well. For the few who have not and are still using paper claims, it is probably time to become electronic if at all possible.
The idea that your LIS is likely outdated by the time you have implemented it is not so far off in regard to the ICD-10 transition. Because of the differences in the coding digits and alphanumeric combinations, an older LIS may not have the capability and workarounds may need to be used. This is true not only for the LIS and EHR but also for any electronic messaging you may be using for ordering and reporting of laboratory tests.
Talk to vendors/health plans. Here is where the first three steps come together into the next conversation. By now, you have checked out the ICD-10 codebook and cross-walked some or all of the codes you use. Now is the time to have conversations with the partners that help you get paid. This may include your LIS company, your IS shop, and the health plans you work with for reimbursement.
Remember that these groups have been working on the transition with other health care departments for some time. If your lab is a little late to this plan, you are likely to benefit from the earlier conversations, planning, and infrastructure changes. A few suggestions: Do not try to reinvent the wheel if your vendor, IS, and reimbursement partners have a process in place; explore how the process may work for the lab. Do suggest and ask for modifications to the process to fulfill the laboratory’s needs.
For example: Your hospital has been working with pulmonary and radiology departments to load in the new ICD-10 codes for those departments. While each of those departments may have different ICD-10 needs to prove medical necessity for visits, procedures, and tests, the laboratory, as a service provider for both departments, would benefit from having both of those coding menus.
Another example: You have a private local laboratory that services two hospitals. Your LIS does not directly interface with the hospital EHR, at least not yet. Set up meetings with the hospitals’ IS staffs to discuss how ICD-10 transitions are happening there. Work with your LIS vendor to see if it has experience with the same EHR systems. Use this information to see where you might be able to use work that has already been done and to strengthen the infrastructure.
Test your systems and processes. Laboratories may have much more testing to do than other health care departments because they work with all of them. However, as laboratory professionals, we are well suited to testing systems and processes because that’s what we do, whether it is performing routine tests for our patients, analyzing QC results, or validating or verifying new tests.
The following are key questions to answer when testing your systems and processes:
Lab information system: Do ICD-10 code sets fit the fields? Do they translate electronically for billing and reimbursement? Can the LIS maintain the old ICD-9 system as well as the new ICD-10 system?
Billing system: Is it able to receive your codes and load them onto the claim? Can the system transmit claims using your tests?
Clinical customers: For the following questions, consider paper versus electronic orders, internal versus external customers, out-of-state customers, and direct billing customers. Have you discussed the ICD-10 transition with your clinical customers? What have they done in terms of their roadmap? Where are they in the process? Select key tests and a variety of ICD-10 codes for testing purposes.
Reimbursement customers: If you work with a type of billing clearinghouse, this may be a bit easier because it will be doing the same testing with each of its insurance plans. Questions include: Has the billing department tested electronic claims (or paper if appropriate) with each of the insurance plans? Have laboratory claims been part of the testing? Ask for the documentation of the findings, including areas that needed improvement or other changes. If the billing companies have not tested laboratory-based claims or tests that you provide, ask for such tests to be completed so you feel comfortable that your claims will go through smoothly the first time around.
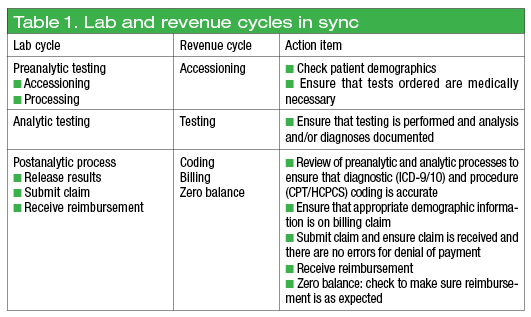
For all testing scenarios, it may be a good idea to test as if it were Oct. 1 or 2. Most of the orders will require the new ICD-10 codes. However, a few stragglers are likely to be collected on Sept. 30 that will require ICD-9 codes. The goal is to follow these orders from accession through to zero balance to make sure that accurate information is passed along at every step in the testing and revenue cycle. Table 1 shows the flow of how the laboratory and revenue cycles match up.
Guidelines. The CMS provides guidelines for the ICD-10 transition: www.cms.gov/Medicare/Coding/ICD10/Downloads/2016-ICD-10-CM-Guidelines.pdf. Reviewing and discussing the guidelines is another article in itself, but having the bookmark and reviewing the chapters will assist you with your roadmap and in walking through the steps.
GEMs. The CMS provides a variety of updates and General Equivalence Mappings, or GEMs, that assist with understanding the ICD-10 transition. Part of the U.S. modification was to provide a direct conversion from ICD-9 to ICD-10. This is likely where the U.S. health care system will start and then, with time, it will continue to expand the codes so they become more and more specific to the health of the patient.
The good news here is that we can use the direct and approximate conversion codes for ICD-10 starting in October. Best practice indicates, however, that we should still follow the basic coding examination, review, and application when applying ICD-10 codes.
Cytopathology laboratory application
ICD-9 to ICD-10 crosswalk. The crosswalk from ICD-9 to ICD-10 is not difficult but it is time-consuming. It can be done electronically or using paper code sets. Each can be bookmarked for use in the future. The goal of the crosswalk is to provide a direct or near direct conversion of an ICD-9 code to a new ICD-10 code.
We still have the same regulations that say the laboratory must perform tests that are medically necessary, so are we really responsible for the crosswalk? In a way, yes. As laboratorians we need to be sure the codes provided are accurate and valid. We must be able to communicate with our clinical partners to provide the most accurate and efficient diagnostic results to our patients. For this reason, we need to understand how the crosswalk works and communicate effectively when the codes are not valid or specific enough for not only quality patient care but also reimbursement for services provided.
It’s important for another reason: A few old ICD-9 codes are also new ICD-10 codes but the descriptions are extremely different. The code may be valid before and after Oct. 1 but will no longer be appropriate for meeting various medical necessity guidelines. The best example from a cytology standpoint is V76.2, the ICD-9 code for a screening Pap test. The description of the ICD-10 V76.2 code is: Person on outside of bus injured in collision with other nonmotor vehicle in nontraffic accident. In the U.S., the ICD-10 V76.2 code will be a non-billable code. However, it may be available in other countries.
In the crosswalk, the same basic guidelines apply as in trying to find the most appropriate code that explains signs and/or symptoms the patient has for a particular visit, procedure, or test. They are as follows:
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
