Amy Carpenter Aquino
February 2019—Two breast cases—one of apocrine carcinoma and androgen receptor overexpression and another of metastatic ER-positive cancer and an ESR1 mutation—were the focus of a molecular oncology tumor board session at CAP18.
Aditya Bardia, MD (MBBS), MPH, breast medical oncologist at Massachusetts General Hospital and assistant professor of medicine, Harvard Medical School, opened the presentation last fall with the first case: a 58-year-old woman with a history of stage I triple-negative breast cancer. Dr. Bardia’s co-presenter was Deborah A. Dillon, MD, breast pathologist, Brigham and Women’s Hospital, and assistant professor of pathology, Harvard Medical School.
Routine screening mammography detected a 1.4-cm tumor in the patient in 2013. There was no noted presence of cancer in nearby lymph nodes. The patient underwent right breast conservation surgery, which revealed a 1.4-cm invasive ductal carcinoma, grade two to three, which was moderately to poorly differentiated. It was “essentially triple-negative breast cancer,” Dr. Bardia said, noting the estrogen and progesterone receptors were zero and the HER2 was 2+ —borderline but with a negative FISH result.
“Given that she had triple-negative breast cancer of more than one centimeter, the plan was made to give adjuvant chemotherapy to reduce the probability of recurrence,” Dr. Bardia said.
During a routine follow-up exam in 2015, it was discovered that the patient had a right palpable supraclavicular lymph node associated with a sore throat, both of which the patient attributed to a viral illness.
“It was large, 2.5 centimeters, non-tender, firm, and somewhat fixed,” Dr. Bardia said. The exam did not find any other palpable lymph nodes in the cervical or axillary region bilaterally, and the rest of the exam was negative. The woman’s lab results were within normal ranges. An ultrasound of the lymph node revealed a highly suspicious 2-cm supraclavicular lymph node, and a CT scan of the neck, chest, and pelvis revealed a 2.6-cm supraclavicular lymph node as well as a lytic lesion in the pelvis and several approximately 1-cm bilateral pulmonary masses.
So what would be the best site to biopsy—lymph node, lung, or bone? While one could consider a bone biopsy, Dr. Bardia said, “from a molecular diagnostic perspective, there’s potential concern with decalcification. From a patient perspective, it’s more difficult to do. And there’s always a concern about missing the lesion. If you get a negative bone biopsy, you cannot completely rely on it being truly negative.” The preference, then, would be to perform a lymph node biopsy (core needle), which is what the patient had.
Does it matter to the oncologist, Dr. Dillon asked, whether distant disease is confirmed as opposed to supraclavicular disease?
“If this was an axillary lymph node,” Dr. Bardia said, “then one could consider a biopsy of a more distant site. But a supraclavicular lymph node would be considered metastatic disease.”
The patient also had BRCA testing because of the likely metastatic disease, for which “one would consider a PARP inhibitor, particularly for germline BRCA mutant triple-negative breast cancer.” A maternal aunt had been diagnosed with postmenopausal breast cancer at about age 60, but there was no family history of ovarian cancer or any other malignancy, Dr. Bardia added. The germline BRCA test result was negative.
Tumor genomic profiling to identify additional actionable alterations was the third consideration. The woman declined out of concern that a discovery of hereditary risk could have family and insurance implications.
The lymph node biopsy results revealed a poorly differentiated invasive carcinoma with apocrine features that was ER/PR-negative and HER2-negative. “You can appreciate the apocrine differentiation, the enlarged nuclei, prominent nucleoli, and granular eosinophilic or foamy cytoplasm,” Dr. Dillon said. “This is an appearance we can see in invasive carcinoma of no special type, but we also can see this apocrine appearance in a number of the special-type cancers, including lobular and micropapillary.”
The differential diagnosis sometimes will include, for example, squamous cell carcinoma, histiocytoid carcinoma, and granular cell tumors. “You may want to do a couple of stains in order to rule those out before calling it a carcinoma with apocrine differentiation,” she said.
Whether the apocrine carcinoma is a specific subtype and whether it matters is another consideration, Dr. Dillon said.
A 2008 gene expression study showed that the morphologic special subtypes, including mucinous, classic, invasive lobular, and micropapillary, could each be classified within a single intrinsic subtype, except for apocrine carcinomas (Weigelt B, et al. J Pathol. 2008;216[2]:141–150). “It is interesting because the apocrine carcinomas live with the pleomorphic lobular carcinomas in a group that has been called molecular apocrine, a subtype of triple-negative cancer,” Dr. Dillon said.
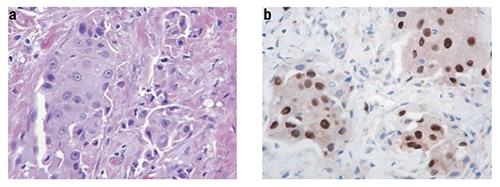
A triple-negative (ER negative, PR negative, and HER2 negative) apocrine tumor (a; × 400, H&E) showing strong AR reactivity (b; × 400, anti-AR)
Reprinted by permission from Springer Nature: Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol. 2010;23(2):205–212. doi: 10.1038/modpathol.2009.159
Because of this overlap in the gene expression studies, the World Health Organization has not classified invasive carcinoma with apocrine differentiation as a special subtype. Survival for the non-apocrine invasive cancers in most studies is similar to that of apocrine carcinomas, which supports the idea that it is not a special subtype, Dr. Dillon said.
“However, it does have a very characteristic biologic profile that is ER/PR negative, androgen receptor positive, and GCDFP15 positive. So we think this is best considered at this time to be a distinct morphologic variant of invasive cancer that has a characteristic gene expression profile. But this unique profile may represent a distinct biologic entity, and we expect to see more research activity in this area moving forward.”
Most important is that it suggests an alternative treatment strategy. “This is not just pathologist navel-gazing,” Dr. Dillon said, noting that the patient’s cancer was strongly androgen receptor positive at 80 percent. “We are talking about possibly doing androgen receptor blockade in these cancers.”
Does androgen receptor positivity influence the choice of first-line chemotherapy, and given the AR positivity, are there other targeted therapies to consider? Dr. Bardia introduced data from the TNT trial in which patients with metastatic triple-negative breast cancer were randomized to receive a taxane-based chemotherapy, docetaxel, versus a platinum-based chemotherapy, carboplatin (Tutt A, et al. Nat Med. 2018;24[5]:628–637).“Overall there was no difference in progression-free survival between carboplatin and docetaxel,” Dr. Bardia said.
The study also included genomic sequencing to look at gene expression based on the PAM50 test (Prosigna). “That is where you could really see a difference,” he said. In basal-like tumors, carboplatin was associated with better progression-free survival as compared with docetaxel, while in non-basal tumors, carboplatin had much less activity. Based on those results, one would consider docetaxel over carboplatin for a patient with an androgen receptor-positive, likely non-basal tumor, he added.
“In terms of targeted therapy, the drug enzalutamide blocks the androgen receptor.” It is FDA approved for prostate cancer, Dr. Bardia said, and was also evaluated in a phase two, single-arm trial for patients with triple-negative breast cancer and demonstrated a modest progression-free survival of about three to four months in patients who had received prior treatment.
“The authors then looked at the androgen receptor by a signature that was developed by the sponsor [Medivation],” he said. The androgen receptor-positive signature was associated with better outcomes with enzalutamide compared with the androgen receptor-negative signature. Medivation was sold to Pfizer and it’s unclear, he said, if Pfizer will proceed with the plan for a confirmatory phase three trial.
The patient in the case enrolled in a clinical trial with an oral androgen receptor antagonist and remained on treatment for six months. “If you go back to whether the androgen receptor findings were actionable, in this case it helped triage the patient into a clinical trial with an oral androgen receptor antagonist,” he said.
Dr. Dillon pointed out that there is no standard test for predicting response to androgen receptor targeting: “It often seems to be the case that clinicians are getting ahead of us in terms of treating patients on clinical trials before we have even established our optimal cut points and figured out the best way to do this.”
Data from breast cancers that developed in women enrolled in the Nurses’ Health Study found more than 75 percent of breast cancers were positive for androgen receptor by immunohistochemistry (Collins LC, et al. Mod Pathol. 2011;24[7]:924–931). “This includes the large majority of estrogen receptor-positive tumors, more than half of HER2-positive tumors, and about a third of triple-negative breast cancers,” Dr. Dillon said, noting that most of the interest in androgen receptor targeting is in the triple-negative breast cancers.
Numbers will vary based on antibody, dilutions, and cutoff points, she said. The Nurses’ Health Study used the Dako AR441 antibody at 1:200 with a one percent cutoff.
Two recent phase two clinical trials used a cutoff point of at least 10 percent to try to enrich for patients who were more likely to respond, she said. “This includes the TBCRC011 trial of bicalutamide. When they defined positivity as greater than or equal to 10 percent, 12 percent of triple-negative breast cancer patients screened were positive for androgen receptor.”
A recently published phase two enzalutamide trial also defined AR positivity as greater than or equal to 10 percent. Fifty-five percent of the patients screened were positive for AR by that definition (Traina TA, et al. J Clin Oncol. 2018;36[9]:884–890). Two antibodies were used in the trial, Dako AR441 and Ventana SP107, and concordance was high.
“The best cutoff point to use in the selection of patients for treatment with an androgen receptor inhibitor is not known,” Dr. Dillon said. “We also don’t know what the significance is of other androgen receptor alterations, such as amplification and mutation, which of course we are starting to see now that we’re sequencing tumors.”
Amplification would be expected to lead to overexpression of the proteins, so that is a “pretty straightforward lead that amplification is going to predict response,” she said.
Mutations, in some cases, abrogate the function of the androgen receptor. “There’s a suggestion, at least in the literature, for prostate. There’s report of a missense mutation in androgen receptor that conferred enzalutamide resistance, so there is certainly more work to be done here.”
Dr. Dillon
At Brigham and Women’s Hospital, pathologists report the apocrine morphologic features in apocrine cancers. “We think this is helpful in the recognition of recurrences and metastases. It’s also a tipoff to our oncology colleagues if they want to consider the patient for androgen receptor testing.” They don’t routinely do androgen receptor testing but will do so at the oncologist’s request if a trial is being considered. “When we do androgen receptor testing,” Dr. Dillon said, “we don’t report it as positive or negative; we just report the percent positive tumor cells and leave the trial eligibility decision to the oncologist.”
In summing up, Dr. Bardia said triple-negative breast cancer is a heterogeneous disease in molecular profile as well as drivers. About 55 percent of patients with triple-negative breast cancer had an androgen receptor of more than 10 percent staining by IHC in the phase two enzalutamide trial, “but it should also be noted that there was a lot of patient selection in the trial. You do have some sense that this could be a patient who has an androgen receptor-positive tumor based on their prior history and age as well as response to a prior chemotherapy agent. I suspect that is why 50 percent of patients had an androgen receptor of more than 10 percent in this clinical trial.”
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
