March 29, 2024—Nova Biomedical announced the U.S. launch of its 510(k)-cleared, next-generation StatStrip Glucose Hospital meter system.
Read More »FDA clears Nova Prime Plus for microcapillary sample mode
November 2023—Nova Biomedical announced that the FDA has granted 510(k) clearance for a microcapillary sample mode on the company’s Stat Profile Prime Plus critical care blood gas analyzer.
Read More »FDA clears Nova Prime Plus for microcapillary sample mode
Oct. 5, 2023—Nova Biomedical announced that the FDA has granted 510(k) clearance for a microcapillary sample mode on the company’s Stat Profile Prime Plus critical care blood gas analyzer. It is available as a standard feature.
Read More »FDA clears Nova’s blood glucose reference analyzer
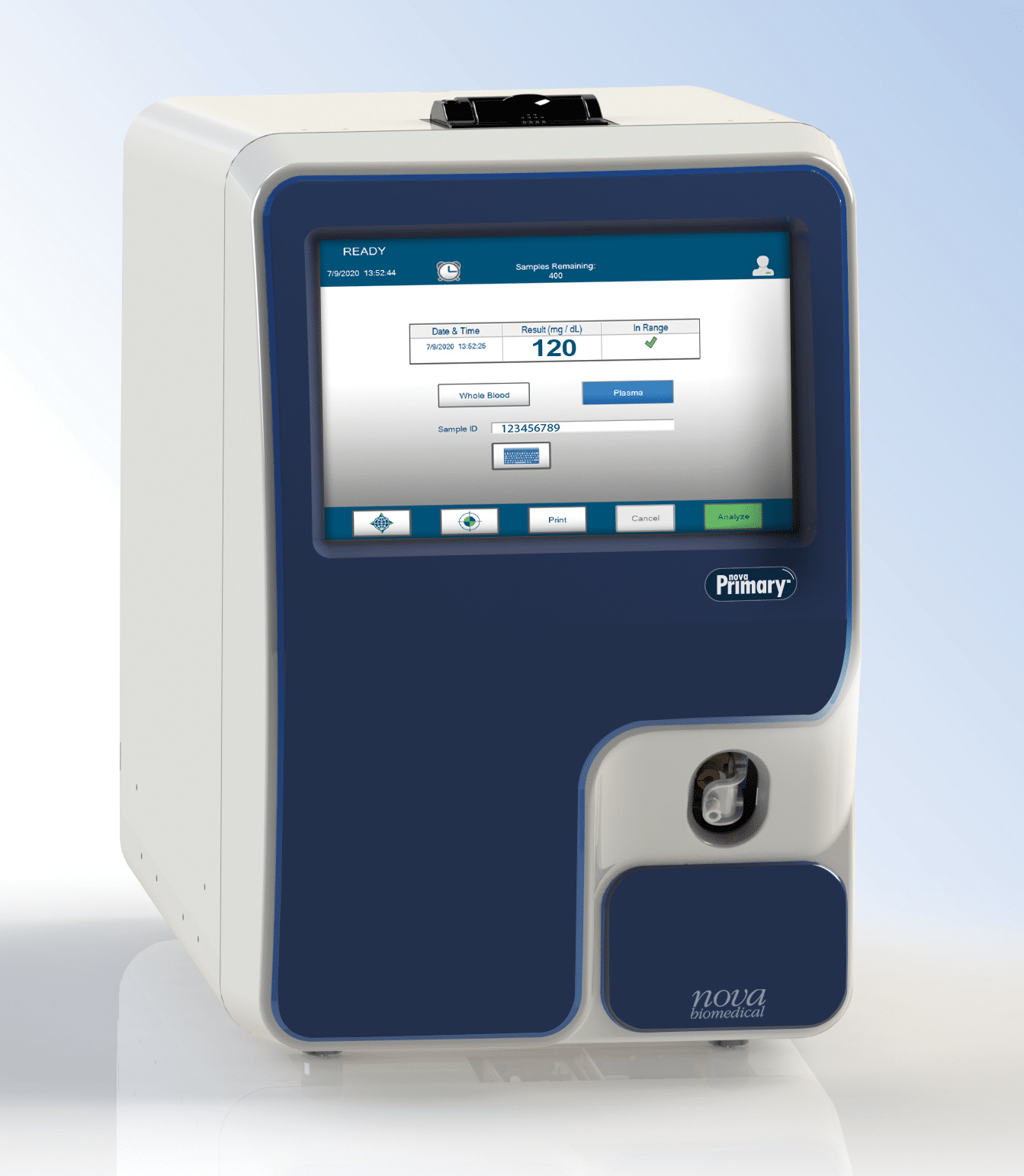
December 2022—The FDA has cleared Nova Biomedical’s Nova Primary blood glucose reference analyzer. Nova Primary is designed to replace the YSI Stat Plus 2300 Glucose and L-Lactate analyzer, which has been discontinued by YSI.
Read More »CE-marked Nova Max Pro creatinine/eGFR meter launched in EU
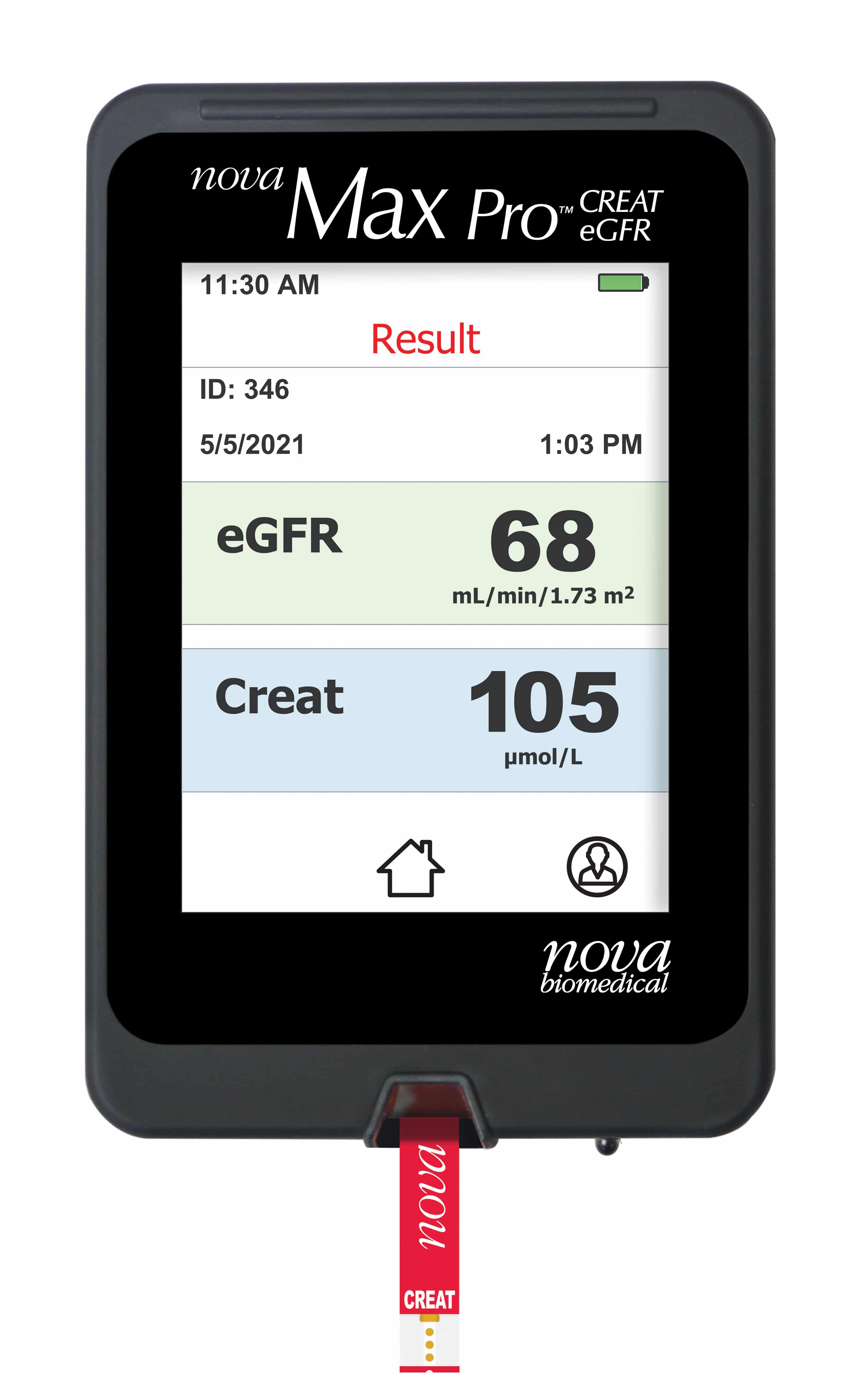
June 2022—Nova Biomedical launched the CE-marked Nova Max Pro creatinine/eGFR meter system in the EU market. Nova Max Pro is designed to improve kidney care through kidney function screening and early detection of kidney disease in point-of-care settings outside the hospital. The meter and creatinine biosensor measure blood creatinine and calculate estimated glomerular filtration rate from a 1.2-μL capillary fingerstick blood sample in 30 seconds. The measuring technology is based on the Nova StatSensor Creatinine technology.
Read More »CE-marked Nova Max Pro creatinine/eGFR meter launched
March 11, 2022—Nova Biomedical launched the CE-marked Nova Max Pro creatinine/eGFR meter system in the EU market.
Read More »Nova Biomedical blood glucose reference analyzer
January 2021—Nova Biomedical announced the availability of Nova Primary, a rapid blood glucose laboratory analyzer. Nova Primary fills the need for a glucose reference analyzer to replace the YSI Stat Plus 2300 Glucose and L-Lactate analyzer, which, as of July 2021, will no longer be supported by YSI, Inc.
Read More »Nova Biomedical launches blood glucose reference analyzer
Nov. 13, 2020—Nova Biomedical announced the availability of Nova Primary, a rapid blood glucose laboratory analyzer.
Read More »Nova webinar on COVID-19 bedside glucose management
May 2020—Nova Biomedical announced a webinar, titled “COVID-19 Bedside Glucose Management: Risk of Ascorbic Acid and Hematocrit Interference,” to help inform and support health care workers treating COVID-19 patients. The webinar, led by Charbel Abou-Diwan, PhD, director of Nova Biomedical’s medical and scientific affairs, examines the risk of inaccurate glucose meter results due to interference from ascorbic acid and anemia.
Read More »Nova webinar on COVID-19 bedside glucose management
April 20, 2020—Nova Biomedical announced a webinar, titled “COVID-19 Bedside Glucose Management: Risk of Ascorbic Acid and Hematocrit Interference,” to help inform and support health care workers treating COVID-19 patients. Led by Charbel Abou-Diwan, PhD, director of Nova Biomedical’s medical and scientific affairs, the webinar examines the risk of inaccurate glucose meter results due to interference from ascorbic acid and ...
Read More »Stat Profile Prime Plus FDA cleared for POC use
April 15, 2020—Nova Biomedical announced the FDA has cleared its Stat Profile Prime Plus critical care blood gas analyzer for point-of-care use. This clearance allows POC personnel to perform bedside critical care testing with results in one minute.
Read More »Nova Biomedical adds PT/INR test to Allegro
April 8, 2020—Nova Biomedical has added PT/INR testing to its Allegro capillary blood analyzer for point-of-care testing in primary care settings.
Read More »Nova Biomedical introduces electrolyte analyzer
December 2019—Nova Biomedical announced the release of its Stat Profile Prime ES Comp Plus. The analyzer offers a complete electrolyte profile, including ionized magnesium, and optional serial batch testing on whole blood, serum, or plasma.
Read More »Nova Biomedical introduces ES Comp Plus Electrolyte System
Sept. 18, 2019—Nova Biomedical announced the release of its Stat Profile Prime ES Comp Plus. The analyzer offers a complete electrolyte profile, including ionized magnesium, and optional serial batch testing on whole blood, serum, or plasma.
Read More »Nova Biomedical awarded group purchasing agreement
March 2019—Nova Biomedical has been awarded a multiyear group purchasing agreement for critical care blood gas analyzers from Premier. The agreement allows Premier members to take advantage of special, prenegotiated pricing and terms for Nova’s Stat Profile Prime Plus critical care blood gas analyzers and consumables in addition to Nova’s 10-test Prime.
Read More »StatStrip cleared for capillary testing
with critically ill
October 2018—Nova Biomedical’s StatStrip Glucose Hospital Meter System has been cleared by the FDA for fingerstick capillary testing with critically ill patients. The FDA granted this 510(k) clearance to StatStrip after extensive prospective and retrospective studies were performed.
Read More »Nova Biomedical opens subsidiary in Switzerland
June 2018—Nova Biomedical has opened a sales and after-sales support subsidiary in the canton of Zug, outside of Zurich.
Read More »Capillary blood analyzer for POC testing, 5/17
May 2017—Nova Biomedical received the CE mark for its Allegro, a capillary blood analyzer for point-of-care testing in primary care settings. Allegro and its StatStrip A companion meter provide 14 clinically important tests to monitor glycemic control, assess cardiac risk with a full lipids panel, and assess kidney function. Results are ready during the patient visit.
Read More »Glucose meter cleared for ICU, 8/15
The FDA has cleared Nova Biomedical’s StatStrip Xpress Glucose Hospital Meter System for use throughout all hospital and all professional health care settings, including among critically ill patients. StatStrip Glucose and StatStrip Xpress Glucose are, at press time, the only two hospital blood glucose meters to be cleared by the FDA for use with critically ill patients. StatStrip Xpress Glucose uses the same test strip measurement technology as StatStrip Glucose, which was cleared in 2014 after a four-year study conducted at five university medical centers.
Read More »Next-generation blood gas analyzer, 6/14
May 2014—The Stat Profile Prime is a blood gas analyzer from Nova Biomedical. It combines the company’s MicroSensor Card with its Zero maintenance cartridge technology, which consists of individual cartridges for biosensors, calibrators, and liquid quality control.
Read More » CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management