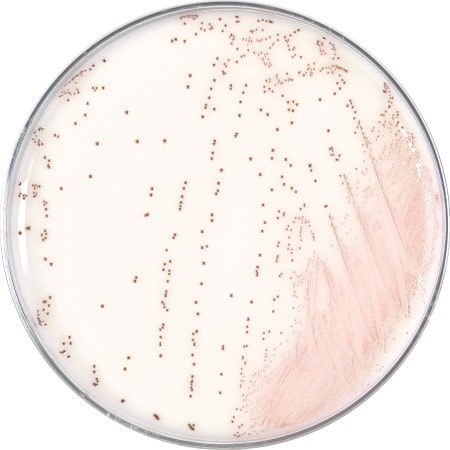
December 2021—Hardy Diagnostics has released its HardyChrom Candida plus auris, a chromogenic media recommended for the selective isolation and differential identification of Candida species. This medium allows for the identification of C. albicans, C. tropicalis, and C. krusei, and the presumptive identification of C. glabrata.
Read More »Hardy Diagnostics releases group A strep agar
November 2021—Hardy Diagnostics released its HardyChrom Group A Strep agar, a chromogenic medium recommended for the selective cultivation and differentiation of group A streptococcus from clinical specimens. Identification can be made based on colony color—the medium will display red-brown or red-orange colonies on the HardyChrom Group A Strep agar when Streptococcus pyogenes is present; non-group A streptococcus isolates will appear as blue, clear, or white colonies.
Read More »Hardy Diagnostics releases HardyChrom Candida + auris
Oct. 21, 2021—Hardy Diagnostics has released its HardyChrom Candida + auris, a chromogenic media recommended for the selective isolation and differential identification of Candida species.
Read More »Hardy Diagnostics releases group A strep agar
Aug. 24, 2021—Hardy Diagnostics has released its HardyChrom Group A Strep agar, a chromogenic medium recommended for the selective cultivation and differentiation of group A streptococcus from clinical specimens.
Read More »FDA grants Hardy Diagnostics EUA for COVID-19 antibody test
May 5, 2020—Hardy Diagnostics announced FDA EUA approval the Anti-SARS-CoV-2 Rapid Test.
Read More »Hardy Diagnostics releases rapid test for COVID-19
March 30, 2020—Hardy Diagnostics announced it is a U.S. supplier of the Anti-SARS-CoV-2 Rapid Test, developed by Autobio Diagnostics (Zhengzhou, China).
Read More »Hardy Diagnostics, EliTech partnership
April 2019—Hardy Diagnostics is now an authorized distributor of EliTech’s Mycofast US, a rapid system to detect, enumerate, and identify genital Mycoplasma hominis and Ureaplasma urealyticum.
Read More »Selective and differential medium, 1/17
January 2017—Hardy Diagnostics announced its new product, C Diff Banana Broth, which is recommended as a selective detection broth for the recovery of Clostridium difficile from environmental samples. Its name is derived from the color change reaction when C. diff is present; the broth changes from a deep red, indicating a negative reaction, to a milky yellow, indicating a positive reaction.
Read More »Hardy Diagnostics acquires GG&B, 4/16
April 2016—Hardy Diagnostics announced the acquisition of Wichita Falls, Tex.-based GG&B Company, maker of automated microscope slide stainers for laboratory use.
Read More »Tuberculosis test kit
January 2015—The TB MODS Test Kit, from Hardy Diagnostics, uses the microscopic observation of drug susceptibility (MODS) method to simultaneously detect Mycobacterium tuberculosis and the strain’s resistance to two common tuberculosis antibiotics—isoniazid and rifampicin.
Read More »Medium for detecting enteric pathogens, 8/13:88
The HardyChrom SS, from Hardy Diagnostics, is a selective chromogenic medium recommended for use as a primary screening medium for isolating and differentiating Salmonella and Shigella spp from stool cultures.
Read More »Media-fill kits for USP 797 testing, 5/13:88
Hardy Diagnostics’ Val media-fill challenge kits are for USP 797 compliance testing for compounding-sterile preparations (CSPs). The ready-to-use media-fill challenge kits are for use in validating pharmacy compounding personnel in accordance with USP 797 guidelines. Aseptic media-fill testing is part of an effective quality assurance program to ensure pharmaceutical processes and personnel produce sterile products that are free of microbial contaminants.
Read More »Parasite suspensions for QC, 5/13:85
Hardy Diagnostics’ parasite suspensions are designed for a variety of parasitology applications to provide reliable and consistent quality control results. The suspensions are microorganism preparations designed to mimic patient samples to support quality assurance programs in documenting process and detection proficiency. The suspensions can be used in the QC or validation of diagnostic test kits and methods, in microscopic examinations, in staining procedures, in proficiency testing, and for teaching purposes in educational settings.
Read More »Arcanobacterium selective agar, 3/13:74
Hardy Diagnostics’ Arcanobacterium selective agar is a selective medium for the isolation of Arcanobacterium haemolyticum, especially from respiratory specimens.
Read More » CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management