Karen Titus
July 2018—Breast cancer has a way of deflating conventional wisdom:
- Maybe it’s not about the journey.
- Maybe more is better.
- Maybe communication is overrated.
Take the new ASCO/CAP guideline for HER2 testing (Wolff AC, et al. Arch Pathol Lab Med. Epub ahead of print May 30, 2018. doi:10.5858/arpa.2018-0902-SA). Since the first groundbreaking joint guideline appeared 11 years ago, the authors have made a habit of addressing cases that flummox pathologists, medical oncologists, and patients. Now, in 2018, they have clarified the diagnostic approach to in situ hybridization groups two, three, and four, rare cases that nonetheless cause an outsized share of headaches and worries. It also clarifies language from the 2013 guideline that had sent some labs astray, and it addresses the use of multiple alternative chromosome 17 probe assays.
![As Dr. Kimberly Allison puts the new HER2 guideline into practice at Stanford, she’s revising reporting templates and meeting with colleagues in the cytogenetics lab. “In our reporting,” she says, “we want to reflect the additional workup and that some of these result categories are unusual.” [Photo: Cindy Charles]](https://captodayonline.com/wordpress/wp-content/uploads/2018/07/Dr.Kimberly-Allison-1-square_CONV.jpg)
As Dr. Kimberly Allison puts the new HER2 guideline into practice at Stanford, she’s revising reporting templates and meeting with colleagues in the cytogenetics lab. “In our reporting,” she says, “we want to reflect the additional workup and that some of these result categories are unusual.” [Photo: Cindy Charles]
As the guidelines evolved, from 2007 to 2013 and now most recently, “We have learned a lot more about how HER2 testing happens in daily practice,” says Dr. Wolff, who, along with coauthor Elizabeth Hammond, MD (respectively, the lead oncologist and lead pathologist authors on all three guidelines), spearheaded the first document. That, in turn, enabled the current guideline’s authors to refine their focus.
Did someone say Daniel Craig?
Because of the broad reach of earlier documents, the authors wanted to present not only updates—obviously part and parcel of any revised guideline—but also a clear overview of the topic. They did so in the summary tables and with comprehensive figures. “We didn’t just want to say, ‘This is what we changed from last time—read the old document again,’” says coauthor Kimberly Allison, MD, professor of pathology, director of pathology residency training, and director of breast pathology and breast pathology fellowship, Stanford University School of Medicine. “We’re building on too many different things.” Dr. Allison became involved at the tail end of the 2013 update, initially in the role of patient advocate (she notes she was treated for HER2-positive breast cancer in 2008); she’s subsequently been involved because of her professional expertise.
In that sense, the guideline emphasizes what hasn’t changed. “Table 1 is an important read,” Dr. Allison says, “because it has all that laid out,” showing the summary of all recommendations, both the original and focused updates. Moreover, a number of the figures sketch out diagnostic testing algorithms, “which have become somewhat complicated for certain groups of FISH testing.”
The guideline could be summarized in the vein of the oft-recited political poem (albeit with a much happier result):
First they came for the false-positives,
Then they came for the false-negatives,
Now they come for the unusual results cases.
With the first guideline, says Dr. Wolff, “Our main concern was about an excessive number of false-positive test results,” which had become apparent with the enrollment of patients in the first generation of adjuvant trials in HER2-positive disease. Rates ran between 25 and 30 percent. “We were in that moment concerned about the need to improve specificity.”
Having succeeded in reducing the frequency of such results, “We then wanted to make sure we were not potentially missing false-negatives,” he says. The swinging pendulum—between false-positives and false-negatives, specificity and sensitivity—explains the focus the authors took with the 2013 guideline in handling difficult cases. False-negative results were estimated by some to be as high as 10 percent, though the actual frequency was difficult to estimate because only the positive cases were being submitted for central test confirmation.
Laboratories have made significant strides, particularly with preanalytical (care of specimens) and analytical (assay standardization) issues. At this point, “We may have succeeded in improving the accuracy of HER2 testing to above 95 percent,” Dr. Wolff says.
Now, in 2018, the focus has shifted once again, as more information has emerged about less common types of HER2 test results, as seen in dual-probe in situ hybridization (ISH) groups two, three, and four.
“We also began to realize that some labs were misinterpreting some of the recommendations from 2013,” he says. The most obvious example involved cases of reference labs that performed only in situ hybridization. When some of those results were initially inconclusive, labs might re-test with up to five alternative chromosome 17 probes, leading to a significant concern about false-positives, says Dr. Wolff.
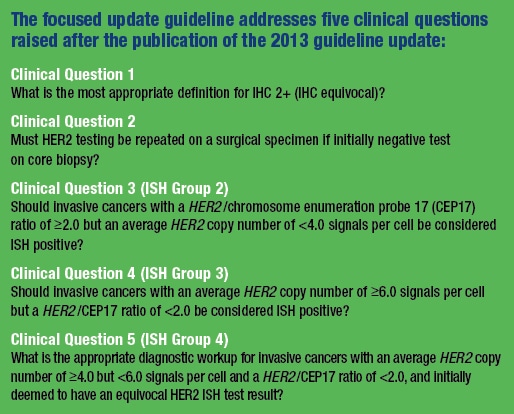
The most recent update ponders five clinical questions, three of which focus on so-called unusual results categories. These are the low-frequency, high-intensity cases—about five percent overall—that, like the piccolo in Beethoven’s 9th, command attention despite appearing only briefly.“We now have very specific guidance that takes into account primarily a joint review of immunohistochemistry and in situ hybridization,” says Dr. Wolff, which “will help pathologists best resolve those difficult cases.”
It comes at a compelling juncture. He and his clinical colleagues are starting to see that “in the treatment of HER2-positive disease, outcomes are improving in a major way.”
Dramatic evidence of this appears in the APT study (Tolaney SM, et al. N Engl J Med. 2015; 372[2]:134–141), which looked at treatment for patients with anatomic low-stage, HER2-positive disease, a group that had been ineligible for the pivotal trials of adjuvant trastuzumab. Patients received weekly paclitaxel and trastuzumab treatments for 12 weeks, followed by nine months of trastuzumab monotherapy. (Endocrine therapy was added in ER-positive cases.) The strategy of deescalating chemotherapy plus trastuzumab from two to three cytotoxic drugs to just one worked, Dr. Wolff says. The study has since been updated—seven-year data is available but not yet published, he says. “The survival in that initial paper [median follow-up: four years] was excellent, and we are now able to offer the potential of excellent survival outcome to patients treated with HER2-targeted therapies while avoiding excessive chemotherapy toxicity,” says Dr. Wolff, a coauthor of the study.
But for deescalation to work, “we need to be cautious,” he says. “We need to have as much certainty about the accuracy of the HER2 testing as possible.” If a candidate patient actually has triple-negative disease, rather than HER2-positive/estrogen-receptor-negative disease, chemotherapy regimens with two or three cytotoxic drugs would be the recommended adjuvant regimen, without any anti-HER2 targeting drugs like trastuzumab.
It’s possible, says Dr. Wolff, looking even further ahead, that deescalation might benefit patients with higher anatomic stage cancer. Patients who have meaningful response to perioperative therapy, who at the time of surgery have evidence of a pathologic complete response, might benefit from an approach similar to that used in the APT study. “In the next generation of HER2-target trials, we plan to integrate pathologic response to neoadjuvant therapy as a functional prognostic biomarker of outcome along with HER2 testing as a predictive biomarker of response to targeted therapy, and design studies that better enrich patients for therapy deescalation or escalation strategies, and ultimately be able to offer just the right treatment for the right patient.”
 “So we really need to make the diagnosis as accurate as possible,” Dr. Wolff reiterates. He sends an approbative look toward the laboratory. “I think we are there.”
“So we really need to make the diagnosis as accurate as possible,” Dr. Wolff reiterates. He sends an approbative look toward the laboratory. “I think we are there.”
That’s not an assumption the guidelines have ever taken lightly, the authors say. And as Dr. Allison notes, “All our same rules apply for test validation and proficiency testing, including fixation and preanalytical variable controls. Those haven’t changed. All those recommendations from the 2013 update still stand.”
With the new guideline, “We actually bring IHC testing into more prominence now that the quality control has gone up. Protein expression is a very important aspect of testing,” she says. “It’s really the phenotype you’re treating, which has a very tight association with HER2 gene amplification.”
While in situ hybridization testing is used by some laboratories as an initial test, most use it as a second-line test when IHC results are equivocal, says Dr. Allison. “And more and more labs are also doing dual testing—IHC and FISH testing on all cases.
“But that’s not a requirement in any way,” she continues. “Most labs can still follow the algorithm of IHC first.” If the result is equivocal (IHC 2+), subsequent ISH testing should follow. Results will unfold in several directions.
One possibility: getting clearly amplified cases (group-one results) that have elevated HER2 signals per cell (≥4.0), and a HER2/chromosome enumeration probe 17 ratio greater than or equal to 2.0. These are obvious ISH positives that correlate well with higher levels of protein expression by IHC (2–3+). In addition, cases with <4.0 HER2 signals per cell and ratios <2.0 are also obvious negatives (group-five results), and these correlate well with the absence of protein overexpression.
And then there are three groups that make up the gray zone. The 2013 guideline acknowledged their existence and proposed result categories for them, but also acknowledged the very limited to nonexistent evidence of their frequencies and clinical-pathologic features and behavior and leaving group-four results in the “no man’s land,” Dr. Allison says, of an equivocal or unresolved result.
Dual-probe ISH groups two, three, and four are uncommon (less than five percent of all cases) but require precision, like passé simple French verb conjugations. They are addressed in clinical questions three, four, and five in the guideline.Cases in group two (the Sheldons of the world might be irritated that these are addressed in clinical question No. 3) have low HER2 copy number (<4.0 signals per cell) but a HER2/CEP17 ratio ≥2.0 These cases tend to have loss of the centromeric control signal. They also would have been considered eligible for the original clinical trials for HER2-targeted therapy, given their ratio-positive status, so the 2013 guideline considered them positive as well. “We initially didn’t want to exclude them from the therapy option,” Dr. Allison says.
The authors of the new guideline looked at recent data on the rates of concordance between IHC positivity versus negativity. “The findings were that these were largely IHC-negative cases,” Dr. Allison says. They’re frequently ER positive, and they typically don’t have biologic features that would suggest they behave more aggressively than an ER-positive, HER2-negative cancer.
“You would expect a HER2-positive truly amplified, truly protein-overexpressing cancer to behave in a more aggressive way than a HER2-negative cancer that’s ER positive,” she says. But the (admittedly limited) data available from the first generation of adjuvant trastuzumab trials suggest patients in group two didn’t receive much benefit from HER2-targeted therapy. Hence the new guideline’s recommendation: For group-two ISH cases, do a concurrent IHC test. “Some labs might just currently do FISH without looking at protein expression in cases like this,” Dr. Allison explains. IHC is a useful guide for scoring ISH/FISH, she says, particularly because it helps recognize if there is regional heterogeneity, but it also can flag discordant results with unusual ISH result categories.
If the IHC result is 3+ positive, “you can go ahead and result the ISH test as HER2 positive,” Dr. Allison says. If it’s IHC negative (zero or 1+), then it should be called HER2 negative. The guideline provides a suggested comment that can be modified and included in the report to note the aforementioned limitations of the early trials, including the enrollment of only small subsets of group-two cases.
And if the blurriness continues, in the form of a 2+ result? “It’s recommended to double-check the initial in situ hybridization result by having a second observer count at least 20 cells that are in that area of 2+ IHC staining,” she says. The second reviewer should be blinded to the first reviewer’s counts. If the result remains in the group-two category, with a 2+ IHC result, “it’s going to be negative overall result for group-two cases,” she says, and should be noted as such with comment. If the new count moves the result into a different ISH category, the final category should be resolved by internal procedures, the guideline notes.
Dr. Allison characterizes these steps as “a little additional workup.” Labs can choose to adopt the additional ISH counting workup for all group-two cases, if that’s easier for the testing parameters. But at minimum, group-two cases that are IHC 2+ should get a second count. Essentially, these cases—again, ratio-positive, signals-per-cell-negative—are considered positive only if additional IHC testing is 3+ positive.
Clinical question No. 4 addresses cases in group three (cue coughing noises from the overfastidious). These are the opposite of group-two cases, in that they are signals-per-call high (≥6.0) but with a HER2/CEP17 ratio <2.0. Should these be considered ISH positive?Only a few rare cases like this were included in one of the original trials, and only if very high HER2 copy number, Dr. Allison notes, since they were ratio negative. But in the 2013 update these cases were considered positive, based on high copy number alone; the ratio, she says, was originally designed to control for polysomy. That places the picture slightly askew. “So instead of true gene amplification causing protein overexpression, is it a cancer that just has multiple copies of chromosome 17? That might not be a true HER2-positive case was the original thinking during the initial trials.” But more recent data, including studies that looked at scrutinizing other areas of the chromosome, showed that the majority of these cases have co-amplification of large regions of chromosome 17, rather than true polysomy, Dr. Allison says.
“This group was a little more controversial than group two,” she says, given that this is likely a more heterogeneous result category. Some data groups indicated the majority of such cases are IHC negative, with a handful of IHC positives that appeared to have very high-level HER2 amplification. Other groups showed a large number of IHC 3+ cases and high levels of amplification.
“Again, it’s a rare group,” says Dr. Allison, which means these cases will be very sample dependent. “It seems like it’s a mixed group. And that includes some more aggressive features and more frequent ER-negativity. Some of these cases have really high-level HER2 amplification, even though they’re also ratio negative, and they have the positive protein overexpression by IHC to support true HER2-positive phenotype.” Again, the recommendation is for concurrent IHC in the workup of an initial group-three ISH result. A negative IHC means pathologists can call the case negative, although, Dr. Allison adds, “I would also double-check a negative result,” to ensure there were no problems with fixation, decalcification, and so on.
Positive IHC results mean the case can be resulted as positive. “That’s a nice concordant result,” she says.
And for those troublesome 2+ IHC cases? “Again, additional counting by a second observer in the areas that are 2+ staining,” Dr. Allison says. In contrast to group two, because of the evidence supporting that many of these cases have co-amplification of the HER2 and centromeric control signals, the guideline’s authors wanted to give the benefit of the doubt to patients who have 2+ IHC results and high levels of HER2 copy number, but are ratio negative. Cases that are 2+ and 3+ are both considered positive. As with group two, the guideline provides a comment for inclusion in pathologists’ reports. Dr. Allison says she starts her comments by noting the results are unusual/infrequent, and that additional testing was done per the new guideline, to explain what’s been done and how that might differ from the way similar cases were previously handled.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
