Q. How can one wisely apply GATA3 immunohistochemistry as a useful tumor marker in diagnostic surgical pathology?
A. GATA binding protein 3 (GATA3) is a transcription factor, which is important for the development and differentiation of multiple tissue types. In the past few years, it has been introduced to surgical pathologists as a sensitive and specific tumor marker for breast carcinoma and urothelial carcinoma.1-5 However, recent literature revealed a wide spectrum of GATA3 expression among various tumor types and even conflicting results on specific tumors such as renal cell carcinoma and pancreatic adenocarcinoma.6-13 The controversy has generated confusion among some pathologists about the diagnostic value of GATA3 and how it should be used in daily practice. To address these issues, the following three steps are recommended as a practical guidance.- Always check if the stain is optimal. According to NordiQC, an international academic proficiency testing program specializing in immunohistochemistry, the optimal stain for GATA3 should be defined using normal kidney and tonsil as positive and negative tissue controls. In kidney, moderate to strong nuclear staining reaction in virtually all epithelial cells lining the collecting ducts and podocytes in glomeruli must be seen. In tonsil, the vast majority of T-cells in the T-zones and dispersed intragerminal T-cells must show an at least weak but distinct nuclear staining reaction. No staining of B-cells should be seen. A stain is too weak if the aforementioned pattern is not visible. On the contrary, overstaining is indicated if in kidney all epithelial cells lining the renal tubules, Bowman’s capsule, or mesangial cells show diffuse positivity. Similarly, overstaining should be considered if the squamous epithelium in tonsil shows that diffuse strong positivity or cytoplasmic staining reaction is present in any cell type. Overstaining is a potential issue with GATA3 IHC. In fact, some of the conflicting results seen in the literature can certainly be attributed to this artifact.
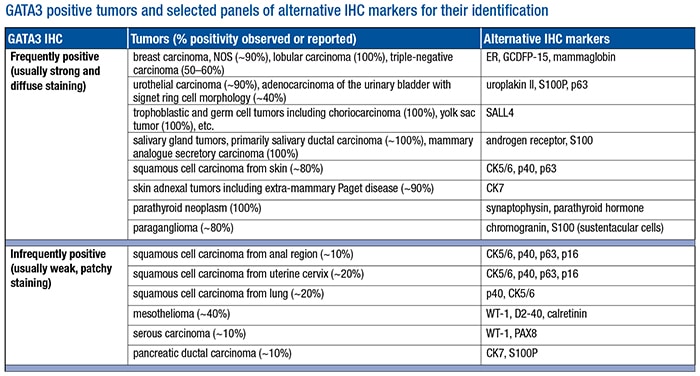
- Be aware of all tumors that may show positivity by standardized IHC. Our laboratory tested more than 1,000 tumor samples using our currently validated protocol (L50-823 clone). We found that the vast majority of tumors tested from the lung, upper and lower gastrointestinal tracts, pancreaticobiliary tract, kidney, prostate, uterus, and ovary were negative for GATA3 expression. The tumors with positive GATA3 staining are listed (see “GATA3 positive tumors and selected panels of alternative IHC markers for their identification”) and can be further divided into two subgroups. The frequently positive tumors consistently show strong and diffuse nuclear positivity in greater than 80 percent of samples tested. The infrequently positive tumors usually show positivity in less than 30 percent of tested samples with weak to moderate staining intensity and focal or patchy distribution. Of note, our data indicate that all renal cell carcinomas including chromophobe type were GATA3 negative, a finding that was also supported by the study published by a group from Johns Hopkins University.12
- Use alternative markers in conjunction to enhance diagnostic confidence. Also listed in the table are selected alternative IHC markers for identifying respective tumor types. They are helpful not only in discriminating GATA3 positive tumors from each other but also in conjunction with GATA3 to enhance its diagnostic sensitivity and specificity in different clinical scenarios. For example, a GATA3 positive poorly differentiated carcinoma can be of either urothelial or breast origin. Adding uroplakin II and mammaglobin or estrogen receptor in this setting can significantly enhance diagnostic confidence. Similarly, instead of using GATA3 alone, a small panel of immunomarkers is usually required in dealing with challenging differential diagnoses such as diffuse type gastric carcinoma versus metastatic lobular carcinoma of the breast, urothelial carcinoma versus squamous carcinoma of the anogenital tract, peritoneal serous carcinoma versus micropapillary urothelial carcinoma, and others.
 In summary, GATA3 is still an extremely valuable tumor marker available currently for identifying breast and urothelial carcinomas and differentiating them from other morphological mimickers. A concise understanding of the GATA3 IHC profile among different tumor types is fundamental for exploring and optimizing its diagnostic value in surgical pathology practice. To achieve the best results, following the aforementioned three steps in clinical practice is recommended. As a matter of fact, the same steps can be generalized for optimizing the diagnostic utility of any immunomarkers.
In summary, GATA3 is still an extremely valuable tumor marker available currently for identifying breast and urothelial carcinomas and differentiating them from other morphological mimickers. A concise understanding of the GATA3 IHC profile among different tumor types is fundamental for exploring and optimizing its diagnostic value in surgical pathology practice. To achieve the best results, following the aforementioned three steps in clinical practice is recommended. As a matter of fact, the same steps can be generalized for optimizing the diagnostic utility of any immunomarkers.
- Higgins JP, Kaygusuz G, Wang L, et al. Placental S100 (S100P) and GATA3: markers for transitional epithelium and urothelial carcinoma discovered by complementary DNA microarray. Am J Surg Pathol. 2007;31(5):673–680.
- Esheba GE, Longacre TA, Atkins KA, Higgins JP. Expression of the urothelial differentiation markers GATA3 and placental S100 (S100P) in female genital tract transitional cell proliferations. Am J Surg Pathol. 2009;33(3):347–353.
- Liu H, Shi J, Wilkerson ML, Lin F. Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol. 2012;138(1):57–64.
- Liu H, Shi J, Prichard JW, Gong Y, Lin F. Immunohistochemical evaluation of GATA-3 expression in ER-negative breast carcinomas. Am J Clin Pathol. 2014;141(5):648–655.
- Cimino-Mathews A, Subhawong AP, Illei PB, et al. GATA3 expression in breast carcinoma: utility in triple-negative, sarcomatoid, and metastatic carcinomas. Hum Pathol. 2013;44(7):1341–1349.
- Schwartz LE, Begum S, Westra WH, Bishop JA. GATA3 immunohistochemical expression in salivary gland neoplasms. Head Neck Pathol. 2013;7(4):311–315.
- So JS, Epstein JI. GATA3 expression in paragangliomas: a pitfall potentially leading to misdiagnosis of urothelial carcinoma. Mod Pathol. 2013;26(10):1365–1370.
- Chang A, Brimo F, Montgomery EA, Epstein JI. Use of PAX8 and GATA3 in diagnosing sarcomatoid renal cell carcinoma and sarcomatoid urothelial carcinoma. Hum Pathol. 2013;44(8):1563–1568.
- Gruver AM, Amin MB, Luthringer DJ, et al. Selective immunohistochemical markers to distinguish between metastatic high-grade urothelial carcinoma and primary poorly differentiated invasive squamous cell carcinoma of the lung. Arch Pathol Lab Med. 2012;136(11):1339–1346.
- Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36(10):1472–1476.
- Compton LA, Buchanan M, Hirsch MS. GATA3 is a sensitive and relatively specific biomarker for testicular choriocarcinoma. Mod Pathol. 2014;27(S2):222A.
- Gonzalez-Roibon N, Faraj SF, Munari E, et al. Comprehensive profile of GATA binding protein 3 immunohistochemical expression in primary and metastatic renal neoplasms. Hum Pathol. 2014;45(2):244–248.
- Ordóñez N, Sahin AA. Diagnostic utility of immunohistochemistry in distinguishing between epithelioid pleural mesotheliomas and breast carcinomas: a comparative study. Hum Pathol. 2014;45(7):1529–1540.
Zongming (Eric) Chen, MD, Director, Immunohistochemistry
Haiyan Liu, MD, Director, Cytopathology Fellowship Program
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
