Charles Fiegl
July 2014—Laboratories now may be saved from draconian penalties, such as loss of a CLIA license and probation periods, for mistakenly sending proficiency test specimens to another facility.
Under new rules published by the Centers for Medicare and Medicaid Services, laboratories have the regulatory relief the CAP advocated during the past decade. The CMS will still severely punish those attempting to cheat on proficiency testing, but laboratories that unknowingly or unintentionally refer PT specimens will face alternative sanctions, according to the regulations.
“We still want to work with CMS to evaluate other scenarios that are the result of ignorance or a mistake and not with intent to cheat,” says R. Bruce Williams, MD, a CAP governor and chair of the CAP Council on Scientific Affairs. “In those instances, we need to make sure the punishment fits the crime.”
For years, revocation of a CLIA certificate and two years of probation for the owner and laboratory director were the penalties for PT referrals regardless of the circumstance. In some instances, the penalties were handed down to laboratories when technicians or technologists just a few months on the job, and following standard operating procedures, sent PT specimens for confirmatory or reflexive testing. The number of unintentional PT referrals grew, jeopardizing patient care and costing hospital systems and other organizations millions of dollars.
In May, the CMS released two regulations addressing PT referrals. The first CMS rule, published in the Federal Register on May 2, outlined the alternative sanctions for PT referrals by implementing provisions in the Taking Essential Steps for Testing Act passed by Congress in 2012 with the support of the CAP. The CAP had advocated leniency and exceptions when violations are less serious, while reserving stiff penalties for egregious conduct. The TEST Act was in response to outrage over levying the most severe penalties for innocent mistakes. For instance, a PT referral caused by an inexperienced employee at a laboratory in Ohio in 2012 led federal lawmakers from that state to push for changes and grant the CMS discretion when it comes to PT referrals.
The second rule, published May 12, modernized PT regulations as part of an effort by the Obama administration to reduce regulatory burdens throughout the federal government. The CAP had advocated and supported the CMS in providing clarification to treat PT sample referrals differently in light of standard operating procedures for patient sample referrals. It also added new definitions the CAP recommended to help clarify when a PT referral should not lead to revocation or two-year probation for the laboratory owner or operator.
While there is now more flexibility, the CMS still says PT samples should never be sent outside the laboratory no matter the circumstance. There always will be a consequence for PT referrals.
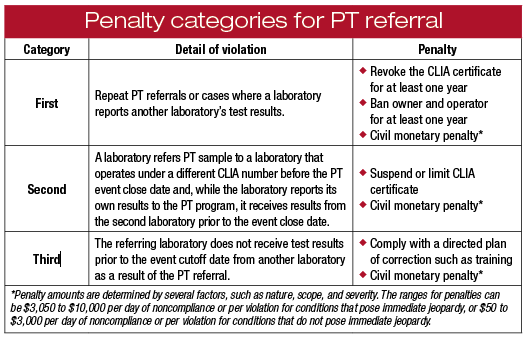
“Whether or not acts are authorized or even known by the laboratory’s management, a laboratory is responsible for the acts of its employees,” the CMS says in the May 12 rule. “Among other things, laboratories need to have procedures in place and train employees on those procedures to prevent staff from forwarding PT samples to other laboratories even in instances in which they would normally forward a patient specimen for testing.”
A narrow exception and alternative sanctions now are options for PT referrals as a result of reflex, confirmatory, or distributive testing. The CMS created specific definitions for these circumstances:
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
