As work in this field unfolds, researchers have made intriguing discoveries. TFE-3 translocation renal cell carcinomas were first identified in children and originally thought to be a pediatric tumor. “Over the years,” Dr. Hirsch says, “we’ve come to recognize this diagnosis in adults, and we now know that we see more cases in adults than in children.” It also appears that behavior and outcome of these tumors differ between the two patient groups as well. In children, the tumors are often confined to the kidney, and survival rates are good. In adults, these tumors not infrequently present as a metastasis before they show up as a primary kidney tumor. They also seem to show up much more frequently in middle-aged women. “I’ve had multiple cases where a TFE-3 translocation renal cell carcinoma presents in a supraclavicular lymph node,” she says. Although Dr. Hirsch can’t explain why this tumor type prefers supraclavicular lymph nodes, she does use this information to her advantage. “If I get a case of a middle-aged woman with a supraclavicular lymph node metastasis and a renal mass, in my mind, that’s a TFE-3 translocated renal cell carcinoma until proven otherwise.”
“In general, we will continue to struggle in a subset of cases where there is morphologic overlap between renal cell cancer subtypes,” Dr. Hirsch says. Without knowing the molecular makeup in all cases, “some of these kidney tumors could very well be misclassified, but this should happen with less frequency as we learn more and more about the genetic and molecular makeup of tumors.”
She and Dr. Reuter sing the same chorus: You can’t diagnose something if you’re not even thinking about it.
Then there’s the matter of clear cell papillary renal cell carcinoma. Channeling her inner Dostoyevsky, Dr. Hirsch says, “It goes by two names.” The WHO recognizes both. One is clear cell tubulopapillary renal cell carcinoma (“That’s the only terminology I use,” she says), which is synonymous with the term clear cell papillary renal cell carcinoma.
Likewise, some pathologists prefer to Type 1/Type 2 their papillary renal cell carcinomas; others (including Dr. Hirsch, Dr. Reuter, and Dr. Merino) do not. Says Dr. Merino: “I’m very opposed to saying something is papillary Type 2. Because that encompasses quite a number of different tumors.”

Dr. Hirsh
Along with everything else, RCC appears to have a branding issue. “Our clinical colleagues do get frustrated,” Dr. Hirsch concedes, “but I think and hope they recognize our good intentions of helping patients get the best possible diagnosis and prognostic information.”
Terminology can eventually change; obviously nothing in this field is static. As noted, sarcomatoid renal cell carcinoma is no longer used; rather, knowledge of genetics and molecular alterations have made clear that tumors with such features arose from one of the recognized RCC subtypes. “We now refer to them as renal cell carcinoma with sarcomatoid differentiation,” Dr. Hirsch says. “And we try to give the underlying subtype, whether we get that from morphology or from genetic and molecular findings: clear cell carcinoma with sarcomatoid differentiation, papillary renal cell carcinoma with sarcomatoid differentiation, etc. But sarcomatoid RCC is not its own subclassification of renal tumors.”
In the case of tubulo versus sans tubulo, Dr. Hirsch says using the latter term is confusing, “obviously because of the clear cell and papillary cell subtypes. The word ‘tubulo’ makes it very distinct in my mind and the clinician’s mind that this is a different subtype.”
Since this subtype was first recognized about five years ago, the cards have been reshuffled a bit, Dr. Hirsch says. The tumor can be separated from traditional clear cell and papillary renal cell carcinomas. And where traditional thinking has considered clear cell renal cell carcinoma as being the most common, followed by papillary, then chromophobe, Dr. Hirsch is convinced that the incidence of clear cell tubulopapillary is higher than that of chromophobe and might even approach that of papillary renal cell carcinoma. “We think of papillary renal cell carcinoma being 10 to 15 percent of renal cell carcinomas; I would say the clear cell tubulopapillary renal cell carcinomas [are] definitely five to 10 percent, if not more, of cases.” The majority of renal cell carcinomas—some 70 percent—are conventional/clear cell RCC.
Pathologists have learned more about the morphologic features of the tubulopapillary tumor, for starters. And whereas clear cell renal cell carcinoma has a chromosome 3p loss, and papillary renal cell carcinomas have extra chromosome copies (i.e. trisomes, often with chromosomes 7 and/or 17), none of these chromosomal changes occur in the clear cell tubulopapillary renal cell carcinoma.
“In prior years, we didn’t even realize this tumor existed,” Dr. Hirsch says. “Now I’m finding this a very frequent tumor. As I go back through my files of renal tumors, I see cases of clear cell tubulopapillary carcinoma that were originally misdiagnoses, and now we see this tumor not infrequently on a routine diagnostic basis. It’s growing in incidence, simply based on recognition.”
While these cases are labeled as carcinoma, Dr. Hirsch says, “We think clear cell tubulopapillary renal cell carcinoma is an indolent lesion. It’s a much better behaving renal cell neoplasm. For that reason, teasing it out is really important. These patients have been dubbed with a carcinoma, and that’s scary for them, and they may lose life insurance, and they may get too many CT scans during routine clinical follow-up. But in the end they really have this very low-grade, indolent, probably benign tumor. And they could have had it taken out and just been told that they’re cured. Which is a completely different emotional situation for that patient.”
Clear cell RCC is anything but clear, as it turns out. “It is a very complex disease,” says Dr. Merino. “And it’s clear we don’t know how to subclassify them according to the genetic changes. It’s possible, with clear cell carcinomas, that we have to do more molecular analysis.” Moreover, she says, “The translocation tumors are tumors that we still don’t know how to recognize very well. Because they have some clear cells, many people would just call them clear cell, when they’re not clear cell. They’re translocation tumors.”
Dr. Reuter offers another example from the TCGA study: Some genomic abnormalities present in chromophobe renal cell carcinomas—tumors that normally have a good prognosis—are associated with poor outcome. “So even within tumors that do well, we can identify features that would predict worse outcome,” he says. “But equally, if those are not present, they predict a good outcome.”
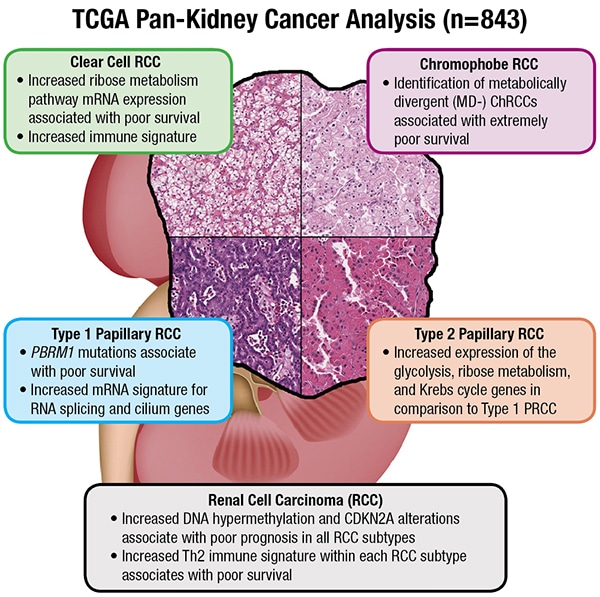
Not to be overlooked in any of this is the importance of pathology, a point the TCGA paper drives home multiple times, says Dr. Reuter. While molecular information would appear to be steering the ship (the words “molecular characterization” are in the title, after all), morphologic context adds a much-needed anchor.Indeed, the Cell Reports paper features, on the opening page, a graphical abstract illustrating RCC subtypes (at right). That was intentional, says Dr. Linehan. “People might say we are entering an era where genomics will make pathology less relevant. That is not the case. The role of the pathologist, armed with new genomic information, is expanding.”
Experienced pathologists, he says, will be invaluable in evaluating the genomic data as well as interpreting histology. “The more we combine those two, the better off we’ll be.” In his view, “The role of the pathologist is even more critical today than it was 15 years ago.”
Dr. Merino agrees, noting that in her quarter of a century of working with Dr. Linehan and others, “It’s been a team effort that has led to successful stories.”
As the complexity of RCC pathology grows, much of the workup is surprisingly within reach of most laboratories.
“You can get pretty far with morphology and immunostains,” says Dr. Hirsch. “But you definitely can’t get to all diagnoses in 100 percent of cases without genetic or molecular information. Even then we still use the ‘unclassified’ category in a small subset of cases. But in this day and age, immunostains are a relatively quick and inexpensive ancillary study that can get to the diagnosis in many of the cases the majority of the time.”
She suggests a “bare bones” group of stains that pathologists could have on hand: PAX8, CK7, CD10, AMACR, CA9, HNF-1beta, S-100A1, CK20, FH (for FH-deficient renal cell carcinoma), and SDHB (for SDH-deficient renal cell carcinomas). “And then I would have a TFE-3 antibody on board, with the caveat that the TFE-3 antibody is very finicky. So I use it with caution. If it’s weak or focal, it’s not contributory and I turn to fluorescence in situ hybridization.” Anything shy of strong and diffuse staining in every single tumor cell is not useful, she says.
“I wouldn’t use these for every case,” she says, “but this group of antibodies would get me to a diagnosis the majority of the time.”
She also recommends that pathologists “go with their gut. If they see a tumor that doesn’t fit into a typical category, they should seek help.”
Despite the intrigue in Anna Pavlovna’s (or is it Annette Scherer’s?) salon, Tolstoy’s story continued to unfold, its characters succumbing to love and loss, disillusionment, revolution and ruin, winter, and many chapters of battle.Dr. Linehan is familiar with the concept of an ongoing saga. Surveying the field of renal cell carcinoma and his 35-plus years of treating patients, he says, “We have an enormous amount of work to do.” What about those four TCGA renal cell carcinoma projects? “It’s a great start,” he says.
Physicians are only beginning to grasp the complexity of hereditary tumors, for example, and pathologists need to be aware that they might be seeing an index case.
Familial tumors, it turns out, are much more common than previously thought, says Dr. Reuter. “It is fair to say that pathologists in the community will confront these cases as specimens without the clinical information that this is a patient with a hereditary syndrome.”
Some morphologies, he continues, are more likely to be associated with hereditary syndromes. There are also findings in the adjacent, supposedly normal renal parenchyma that can point pathologists toward the possibility of a tumor being associated with a hereditary syndrome.
The prototypic hereditary type is VHL, or the von Hippel-Lindau syndrome-related renal cell carcinoma, Dr. Hirsch says. Patients with VHL often develop clear cell renal cell carcinoma, but not everyone with clear cell renal cell carcinoma has VHL—in other words, the VHL gene mutations seen in the kidney tumors can be either germline or somatic.
But VHL is only one of several inheritable syndromes that affect the kidneys. Others include but aren’t limited to MET mutation with hereditary papillary renal cell carcinoma (HPRC); tuberous sclerosis (TSC); the fumarate hydratase-deficient renal cell carcinomas, which are associated with hereditary leiomyomatosis and renal cell carcinoma; and Birt-Hogg-Dubé, which involves an FLCN gene mutation.
These are a frequent topic of discussion with her clinical colleagues, Dr. Hirsch says, and labs are starting to take note. The first step, she says, is to think about the possibility of the diagnosis. If she sees a tumor that she thinks might be an FH-deficient renal cell carcinoma, for example, her next step would be to do a screen with a fumarate hydratase immunostain. “In this case we’re looking for loss of staining of the FH antibody.” If this is indeed the case, “in a note we will tell our clinical colleagues that this patient needs genetic testing to determine if this is a somatic or a germline mutation. And if it’s somatic, they just get treated for their kidney tumor. But if it’s germline, then they need to have their children and family members tested as well.”
 Dr. Linehan seconds that. In the case of HLRCC, “Many times the clinician doesn’t know that’s what the patient is affected with.” This cancer spreads early, when the tumor is still quite small. Knowing the alteration status will change his surgical approach. “It’s more than invaluable; it’s essential,” says Dr. Linehan. “It really is a matter of life or death.”
Dr. Linehan seconds that. In the case of HLRCC, “Many times the clinician doesn’t know that’s what the patient is affected with.” This cancer spreads early, when the tumor is still quite small. Knowing the alteration status will change his surgical approach. “It’s more than invaluable; it’s essential,” says Dr. Linehan. “It really is a matter of life or death.”
A common consult involves tumors with oncocytic cytoplasm, that is, tumors that enter the differential diagnosis of oncocytomas, which by definition are benign tumors. One of the morphologic and molecular limits to identifying this tumor is cytoplasmic eosinophilia. Asks Dr. Reuter: “Have we defined them too strictly in the past, and for that reason not made the diagnosis enough? Or have we used the term too liberally?” Addressing that question will require a combination of better outcomes data and improved molecular findings. “Because what happens now is, if I cannot classify a tumor as an oncocytoma, not put it into any one of those other categories that have eosinophilic cytoplasm, I end up putting it into the ‘unclassified’ category.” This is unpalatable to everyone, he says—clinician, pathologist, and patient.
And so the work continues. Echoing Dr. Linehan, Dr. Reuter takes note both of the ongoing accomplishments and the daunting task ahead. “Putting everything together is very, very difficult,” he says (possibly echoing the words of Tolstoy’s agent after hearing the writer’s War and Peace pitch).
And if it’s not clear by now, the TCGA paper—and related work in the field—is a step, but only a step, toward a better understanding of renal cancer, says Dr. Reuter. “It’s certainly not the end game.”
As readers—both actual and the merely well intentioned—of Russian novels know, the end game is a long one. (This might be a good place to point out that even when War and Peace was over, it wasn’t really over. It concludes with not one, but two—yes, two—epilogues, which add 28 more chapters to the book.)
But somehow even Dr. Linehan can manage to write a simple coda. “I’m very optimistic about the future.”
Karen Titus is CAP TODAY contributing editor and co-managing editor.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
