Michael A. Nalesnik, MD
Aatur D. Singhi, MD, PhD
 December 2016—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from the University of Pittsburgh Medical Center. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
December 2016—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from the University of Pittsburgh Medical Center. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Liver cancer has historically been subdivided into hepatocellular carcinoma and cholangiocarcinoma. Combined types are recognized, suggesting a common cell of origin, and indeed a spectrum of such tumors has been proposed.1 In contrast, primary neuroendocrine tumor of the liver is an extremely rare neoplasm that can be diagnosed only following an extensive search for extrahepatic primary sites, since no markers specific for liver origin of these tumors exist.2 Neuroendocrine tumor is not even listed as a primary liver tumor type by the World Health Organization,3 although some authors recognize a rare neuroendocrine variant of cholangiocarcinoma.4 We present a case in which in situ detection of albumin mRNA strongly suggested primary hepatocellular origin of a liver-based neuroendocrine tumor.
Case. A middle-aged woman underwent ultrasound examination for an unrelated condition and was found to have a 9.5-cm liver mass. No other lesions were identified. The background liver was unremarkable and the tumor was resected surgically. At the time of operation it was felt to represent a hepatocellular carcinoma.
Pathologic examination showed a well-differentiated epithelial neoplasm with a regular appearance and an “organoid” architecture (Fig. A). Given this typical appearance for neuroendocrine tumor, targeted immunohistochemical stains were performed and showed diffuse synaptophysin positivity (Fig. B), with trace PGP9.5 and no chromogranin uptake. A diagnosis of low-grade neuroendocrine tumor on the basis of low mitotic count was made, and the patient continues to do well following surgical resection with no evidence of tumor within or outside the liver to date.
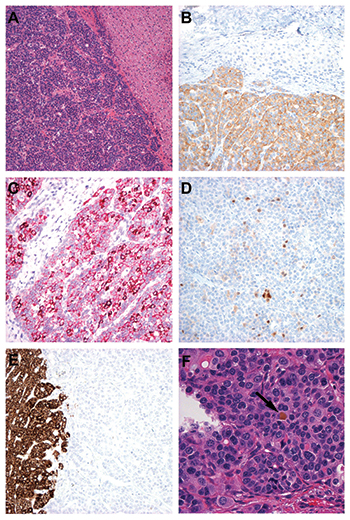
A. The intrahepatic tumor (left) was composed of monotonous small epithelioid cells arranged as nests and sheets with an “organoid” appearance. Background hepatic parenchyma is seen on the right (H&E×10).
B. Uniform synaptophysin uptake (lower half) was present within tumor cells (synaptophysin immunoperoxidase ×20). C. Detection of albumin mRNA by branch chain in situ hybridization showed uniform positivity within tumor cells with areas of hyperintense uptake (albumin branch chain ISH alkaline phosphatase ×20). D. Arginase was present with varying intensity in scattered tumor cells (arginase immunoperoxidase ×20). E. HepPar-1 expression was uniformly absent in tumor cells. Normal liver uptake is seen on the left (HepPar-1 immunoperoxidase ×20). F. A single bile plug (arrow) was detected within the tumor (H&E×40).
Tumor studies. Approximately one year after surgery, tumor sections were randomly selected as negative control tissue for a validation study of albumin RNA ISH assay using the automated Affymetrix branch chain RNA ISH technology. Surprisingly, the tumor showed albumin mRNA expression that surpassed some of the positive samples (hepatocellular carcinoma or normal liver) (Fig. C). This prompted reevaluation of the lesion for additional hepatocellular markers. Arginase-1 (Fig. D) showed patchy uptake in approximately 10 percent of cells with large areas devoid of stain, whereas immunostains for alpha-fetoprotein, HepPar-1 (carbamoyl phosphate synthetase 1) (Fig. E), polyclonal CEA, and CD10 were all negative. Glutamine synthetase, an antigen characteristic of but not specific for hepatocytes, was expressed in about half of the tumor cells. After diligent search, a single small bile plug was found within the tumor (Fig. F).
Discussion. The advent of immunohistochemistry and, more recently, molecular diagnostics has disrupted morphology-based tumor taxonomy by demonstrating common features among tumor types previously considered unrelated. In this case, a tumor diagnosed as neuroendocrine tumor by an experienced pathologist and supported by immunohistochemical stains was serendipitously found to have features associated with hepatocellular origin using a molecular-based approach.
In situ hybridization for albumin mRNA has long been recognized as a marker for benign and malignant hepatocytes. The branch chain RNA ISH technology uses multiple tiling probes that serve as the foundation for hybridization to pre-amplifier molecules. These in turn hybridize to multiple amplifier molecules, each of which attaches to multiple alkaline phosphatase labeled detector probes, leading to a several hundred-fold amplification process. Shahid, et al.5 found this technique to be more sensitive than either HepPar-1 or arginase staining for detecting hepatocellular carcinoma. These authors also reported positivity in intrahepatic, but not extrahepatic, cholangiocarcinoma, suggesting a shared feature that may reflect a common cell of origin. Terris, et al.6 recently challenged this interpretation, maintaining that such positivity may more likely reflect combined hepatocellular-cholangiocarcinoma with stem cell features. Nevertheless, both viewpoints support the concept of plasticity of an intrahepatic tumor progenitor cell able to differentiate in either a hepatocellular or biliary direction.
This case, although a single report, raises the possibility that this plasticity may extend to neuroendocrine differentiation as well. Primary neuroendocrine tumor of the liver is not recognized in the current WHO classification, although neuroendocrine tumors arising in the gallbladder are acknowledged. Identification of neuroendocrine tumors showing specific evidence of primary hepatic origin, if supported by more extensive studies, would not only be of biologic interest but also have direct clinical implications since a diagnosis of primary liver neuroendocrine tumor would preclude extensive radiologic and perhaps surgical exploration in search of a primary tumor elsewhere. Retrospective screening of intrahepatic neuroendocrine tumors for albumin mRNA and arginase-1 in specimens from patients with no recognized extrahepatic tumors is currently underway in our laboratory.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
