Barbara A. Crothers, DO
August 2013—You have a great gynecologic cytology case, a patient with atypical endometrial cells on Pap test that you believe might represent a low-grade endometrial adenocarcinoma, but it has been four weeks and you have had no feedback about the patient’s outcome. It seems as if there have been a lot of atypical endometrial cells on Pap tests lately. Could it be due to the implementation of a new liquid-based technology for Pap tests in your laboratory? Fortunately, your laboratory performs cytologic-histologic correlation monthly, so you ask the medical director if she has noticed any trends in the rate of atypical glandular cells, and what the corresponding biopsies have shown. To your relief, the patient had a biopsy showing low-grade endometrial carcinoma, and the laboratory statistics have shown only a slight increase in atypical glandular cells since the new technology was implemented. The medical director informs you that she has been recording these data as a special QA project to determine if the increase is due to over-interpretation of reactive glandular cells, because the technology enhances nuclear and cytologic details of glandular cells.
What do other laboratories do with cytologic-histologic correlations, and how does it compare with what your medical director does?
The Centers for Disease Control and Prevention in 2010 awarded the CAP a cooperative agreement to investigate, describe, and outline current quality practices in gynecologic cytology, with a goal of establishing standards for common practices and procedures to allow for accurate benchmarking among laboratories. The entire process required months of planning, coordinating with stakeholders, collecting data through surveys (online and mailed), reviewing the literature, and meeting in working groups. In June 2011, the Gynecologic Cytopathology Quality Consensus Conference (GCQC2) was convened, sponsored by the CAP and with the CDC, American Society for Cytopathology, American Society for Clinical Pathology, and American Society for Cytotechnology as partners, to describe and outline effective quality assurance practices in cytopathology, investigate research evidence of effectiveness of specific practices, and gain group consensus for future practices. The proceedings and outcomes are published in a special section of Archives of Pathology & Laboratory Medicine.1
As part of this effort, a Cytologic-Histologic Correlation Working Group was established to investigate measures used for comparing cytologic specimen reports with surgical biopsy outcomes and to review existing literature for the effectiveness of these practices. Although the review focused on gynecologic cytology specimens, the principles for improving current quality assurance practices apply to all cytologic-histologic correlations (CHC).
Cytologic-histologic correlation is a powerful cytopathology quality assurance tool that may be overlooked and underused. For pathologists with limited experience in cytopathology, it is a great educator and feedback mechanism: You get the answer (a biopsy) to your cytologic impression. There are lessons to be learned from both specimens and information to be gained about processes. The elegance of CHC is that regardless of a cytologist’s initial interpretation of a cytology specimen, there is usually a subsequent tissue biopsy that can confirm the result or reveal why the initial interpretation was incorrect. Even though interpretations between observers may vary, the correct interpretation eventually becomes clear. This is in contrast to surgical pathology, where disagreement between individuals over an interpretation may not reveal one correct answer, even though that interpretation is considered the gold standard or “truth” in disease diagnosis. Although this limitation of surgical pathology also affects CHC, in most instances comparing the two specimens is straightforward. Additionally, CHC opens the door for learning experiences in surgical pathology where there are interpretive disagreements about biopsy results.
Cytologic-histologic correlation for gynecologic pathology serves two broad purposes in quality assurance:
- It provides critical information on necessary patient followup by resolving Pap-biopsy discrepancies or confirming discrepant diagnoses as correct, or both.
- It provides a mechanism with which to monitor the performance and processes of the laboratory to improve overall quality.
The CHC Working Group and consensus conference participants came to several broad conclusions about gynecologic cytology CHC. They are summarized here.
Cytologic-histologic correlation may be performed real time or retrospectively or both.
Real-time correlation implies that available cytology slides are reviewed in conjunction with the surgical biopsy or relatively soon after the surgical biopsy is evaluated, but before a surgical biopsy report is issued. The advantage of concurrent review is that it has a greater impact on immediate patient care. It allows the pathologist to provide health care professionals with critical followup information to the cytology in the surgical report and to resolve or discuss discrepancies between the two specimens, if any, in that report. It is strongly preferred in instances where the Pap test is interpreted as high-grade squamous intraepithelial lesion (HSIL) and the followup cervical biopsy is negative, regardless of the outcome of the review of these specimens.
Retrospective correlation is a review of slides after both reports are issued and serves as a monitor of cytology and biopsy performance and processes for laboratory quality improvement. Data collection is more easily performed retrospectively, since computer software can collaborate findings over time in predesigned reports. Retrospective review is logistically more tenable for laboratories with high volumes or that may not have resources for timely concurrent review. Retrospective review is the only option for laboratories that do not receive biopsies on all patients with cytology results from their laboratory.
Bidirectional correlation, or performing CHC real-time and retrospectively, is the most common practice among laboratories, probably because they serve different purposes. Regardless of the method of correlation, results should be reported in a quality assurance document and monitored over time to identify laboratory trends. Results can be monitored weekly, monthly, quarterly, or annually depending on laboratory volume.
At a minimum, review all available slides for high-grade squamous intraepithelial lesion (HSIL) Pap tests with negative biopsies, with a correlation interval between three to four months but not exceeding six months.
Reviewing both the cytology and surgical biopsies for accuracy of interpretation benefits the patient and the laboratory. Review of an HSIL Pap test that reveals erroneous interpretation of metaplastic cells as HSIL could prevent unnecessary procedures. Confirming the original HSIL Pap test result after a negative biopsy is equally important if the health care professional did not find colposcopic evidence of disease, since the lesion may be hidden in the endocervical canal. If the CHC review occurs in real time, pathologists should take all necessary steps to ensure adequate biopsy orientation and leveling to unveil hidden squamous intraepithelial lesion (SIL).
The preferable retrospective search for a Pap test to correlate on a particular patient with a current biopsy is within three to four months, but no longer than six months, from the time of the biopsy. For laboratories that search their databases for retrospective correlation, most patients will have had an incident Pap test resulting in a biopsy within four months. Reviewing Pap tests older than six months from the time of biopsy could result in false-negative results if the lesion evolved or resolved.
For correlation purposes, the “incident” or first prior Pap test with a significant abnormality should be correlated with the most abnormal current tissue obtained. Pap tests taken in conjunction with a biopsy can be excluded from CHC unless the laboratory has no knowledge of the incident Pap test. Additionally, endocervical curettage without cervical biopsies can be excluded unless they contain a squamous or glandular lesion, and excisional biopsies such as loop electrocautery excisional procedures (LEEP), cervical conization, and hysterectomies should be included. Laboratories using HSIL or cancer biopsy targets should correlate with the most abnormal prior Pap test taken within the past six months, and exclude Pap tests taken concomitant with the biopsy unless no earlier Pap test is available.
Laboratories may choose to review Pap tests with potentially less clinically significant lesions, such as atypical squamous cells (ASC), low-grade squamous intraepithelial lesion (LSIL), or reactive changes as a part of CHC when multiple slides are available, but HSIL and cancer are the minimal considerations for review because of the significance of these diagnoses.
Standardization of CHC and its metrics is desirable.
Despite decades of reviewing slides for CHC, there remain no acceptable standards for CHC performance or collection of data metrics. As a result, laboratories have no means of comparing their data with those of their peers. One of the charges of the working group was to offer evidence-based standards for data collection that would allow for peer-to-peer comparison. As result of their literature review, the working group proposed that laboratories monitor the following parameters: 1) the total number of CHC pairs, 2) the number of positive correlations (“true positives,” as defined prior to actual CHC review of the specimens), 3) the number of negative correlations (“false-positives,” as defined prior to review), and 4) the positive predictive value (PPV) of a positive Pap test. These statistics should be tabulated at least annually, although it is appropriate for high-volume laboratories to collect these statistics more frequently.
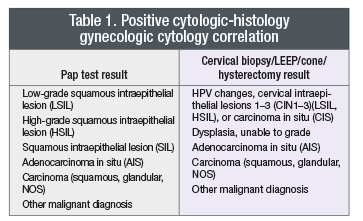 Most laboratories (78 percent of laboratories responding to the survey) already tabulate these statistics, with the exception of the PPV. As a standard, laboratories should use, at a minimum, the definitive of a true positive and true negative as shown in Table 1. If the correlation contains any of the elements from the left and right sides of the table, then it constitutes a positive correlation (abnormality suspected and confirmed). Readers will notice that atypical squamous or glandular cells are not counted in the standard definition of a positive correlation. This does not prevent laboratories from calculating two separate positive predictive values—one with and one without the inclusion of atypical interpretations. Our review of the existing literature on Pap test interpretations of atypical squamous and glandular cells shows very poor inter- and intraobserver concordance, indicating that an atypical Pap test interpretation is not reproducible. This is the primary reason why atypical interpretations were excluded from standardized statistical analysis. A negative correlation is any normal, negative, reactive, or infectious biopsy or Pap test result paired with any interpretation from Table 1.
Most laboratories (78 percent of laboratories responding to the survey) already tabulate these statistics, with the exception of the PPV. As a standard, laboratories should use, at a minimum, the definitive of a true positive and true negative as shown in Table 1. If the correlation contains any of the elements from the left and right sides of the table, then it constitutes a positive correlation (abnormality suspected and confirmed). Readers will notice that atypical squamous or glandular cells are not counted in the standard definition of a positive correlation. This does not prevent laboratories from calculating two separate positive predictive values—one with and one without the inclusion of atypical interpretations. Our review of the existing literature on Pap test interpretations of atypical squamous and glandular cells shows very poor inter- and intraobserver concordance, indicating that an atypical Pap test interpretation is not reproducible. This is the primary reason why atypical interpretations were excluded from standardized statistical analysis. A negative correlation is any normal, negative, reactive, or infectious biopsy or Pap test result paired with any interpretation from Table 1.
The positive predictive value of a positive Pap test is the preferred standard CHC metric, and laboratories should use the PPV for the whole laboratory to formulate QA monitors.
Evidence shows that the PPV is the most reproducible statistic for CHC.2 Most laboratories already collect the data to calculate the PPV but are not aware of the formula to do so. The PPV is defined by the formula:

 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
