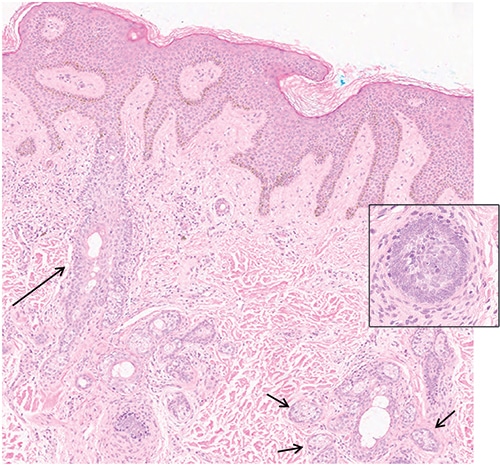
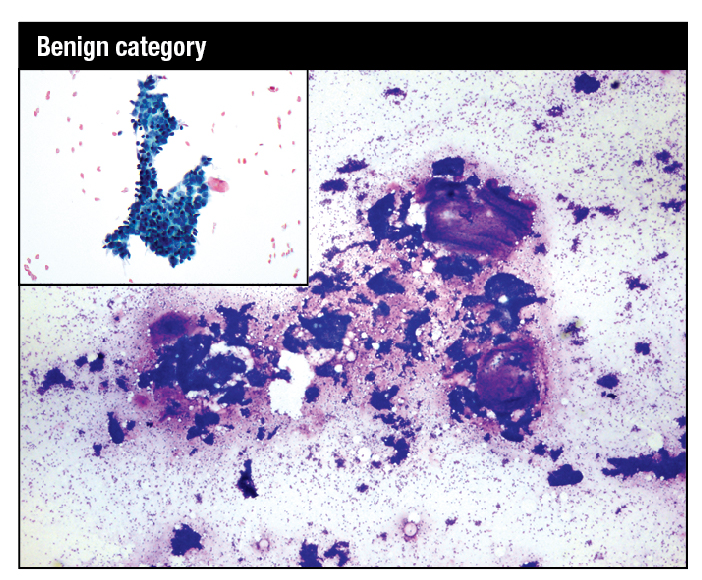
March 2019—Unlike high-count monoclonal B-cell lymphocytosis, low-count MBL has a limited chance of progression to CLL and thus there is no need for follow-up. And a definitive diagnosis of T-cell large granulocytic leukemia requires demonstration of clonality.
Read More »ALL ISSUES
Labs add safety net to critical values procedure
March 2019—A hypercritical value notification policy at Northwell Health in New York State authorizes the laboratory alone to activate a hospital’s rapid response team when necessary. This is the newest step in a critical value notification process that had been working well—but not quite well enough.
Read More »Exploring MALDI-TOF mass spec for mycobacteria
March 2019—The family of Mycobacteriaceae includes the genus Mycobacterium, which includes more than 190 species. These organisms have an unusual cell wall. Those of us who work in mycobacteria laboratories know that the cell wall has a high lipid content that makes these organisms acid fast. Thus they’re acid-fast bacteria.
Read More »Can NGS replace routine respiratory testing? Study says not yet
March 2019—A small study performed at the University of Utah found that a next-generation sequencing assay cannot replace routine standard-of-care testing to detect pneumonia in immunocompromised patients and determine their treatment. But it could be ordered when an infection is suspected and all other testing has failed to find the etiology.
Read More »AMP case report: FDA-approved DNA blood test for colorectal cancer prompts patient to undergo colonoscopy
March 2019—Colorectal cancer is the third most diagnosed cancer and the second highest cause of cancer mortality in men and women, and in 2016 it accounted for about nine percent of all diagnosed cancers in the United States. When CRC is detected at an early localized stage, the five-year survival rate is 90 percent. With progression to regional disease, five-year survival remains high, at 71 percent.
Read More »POC testing roundtable: risks, resources, relationships
March 2019—Infection control and the heavy demands on point-of-care coordinators were among the top concerns that came up in a recent CAP TODAY roundtable on point-of-care glucose testing. Publisher Bob McGonnagle spoke with four POC testing experts: Sharon Geaghan, MD, Cynthia Bowman, MD, Steven Cotten, PhD, and Corinne Fantz, PhD. Here is what they told us.
Read More »Semen analysis guide: a benchtop sperm morphology aid
March 2019—CAP Press released late last year its Semen Analysis Benchtop Reference Guide, an illustrated guide with emphasis on sperm morphology. Its 68 laminated pages are divided into sections: specimen collection and macroscopic assessment, sperm count, sperm morphology, and nonsperm cells. Behind the book is the CAP Reproductive Medicine Committee, in particular Erica J. Behnke, PhD, HCLD, of Ohio Fertility Providers, Jacob F. Meyer Jr., PhD, HCLD, of Eastern Virginia Medical School, and Laura L. Nelsen, MD, pathologist and director of cytology at MaineGeneral Medical Center and past chair of and now advisor to the committee.CAP TODAY asked Dr. Nelsen about the benchtop reference guide, sample pages of which appear below. Here is what she told us.
Read More »Put It on the Board
Lumipulse G β-Amyloid Ratio test has breakthrough device designation
March 2019—Fujirebio Diagnostics received on Feb. 1 breakthrough device designation from the FDA Center for Devices and Radiological Health for its Lumipulse G β-Amyloid Ratio (1-42/1-40) quantitative in vitro diagnostic test. The test uses measurable β-amyloid 1-42 and β-amyloid 1-40 concentrations found in human cerebral spinal fluid and combines those concentrations into a numerical ratio of β-amyloid 1-42/β-amyloid 1-40 to estimate the presence of β-amyloid neuritic plaque pathology in the brain. The Lumipulse G β-Amyloid Ratio (1-42/1-40) combines the results of Lumipulse G β-amyloid 1-42 and β-amyloid 1-40 using the Lumipulse G System. The ratio results are intended to aid in assessing adult patients, ages 50 and over, who present with cognitive impairment and are being evaluated for Alzheimer’s disease and other causes of cognitive decline. The results must be interpreted in conjunction with other diagnostic tools such as neurological examination.
Q&A column
Q. I just read the 2018 HER2 guideline update. Can you provide an example of how a previously equivocal case is resolved under the new guideline? Read answer.
Read More »Newsbytes
Building a lab or modernizing? Don’t forget the following March 2019—Building a new pathology lab or revamping an existing one gives laboratory decision-makers an opportunity to rethink information technology infrastructure and address persistent problems, plan for new technology, and improve processes.
Read More »From the President’s Desk: Our study of member services and support
March 2019—We launched the CAP member services and support strategy two years ago, setting out to figure out which benefits were most valued by the greatest number of members, identify places where we could find better ways to direct or maintain them, and see where we could be falling short. To keep everyone connected and everything on track, we created a coordinating group whose members had access to a fine staff and thoughtfully curated findings from years of member surveys and market research. Our own surveys showed how the interests and needs of our members overlapped. The market provided context.
Read More »Clinical pathology selected abstracts
March 2019—Evaluation of ethanol interference on routine biochemical tests: Ethanol, a central nervous system depressant widely consumed in many societies, is metabolized in the liver through multiple enzymatic pathways. If the liver’s capacity is exceeded, the excess alcohol will flood into the systemic circulation.
Read More »Anatomic pathology selected abstracts
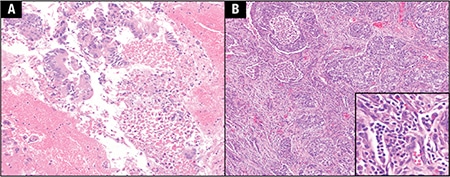
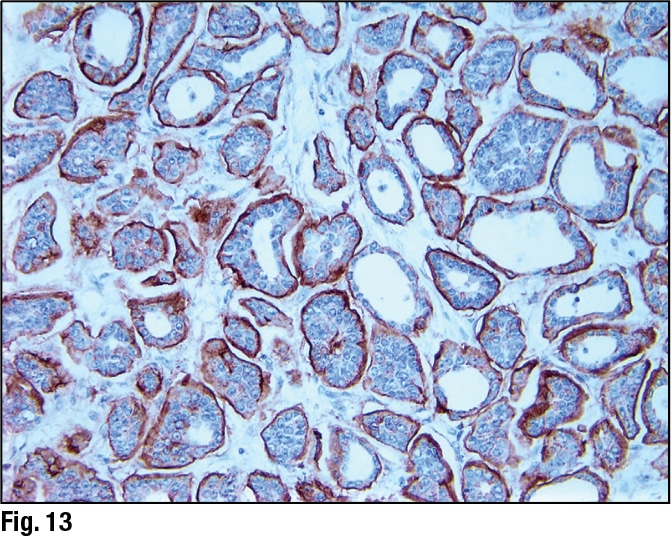
March 2019—ALK expression in angiomatoid fibrous histiocytoma: a potential diagnostic pitfall: The authors recently encountered a case of primary pulmonary angiomatoid fibrous histiocytoma (AFH), which was initially misdiagnosed as inflammatory myofibroblastic tumor based in part on anaplastic lymphoma kinase expression by IHC. Prompted by this experience, they evaluated anaplastic lymphoma kinase (ALK) expression in 11 AFH, 15 inflammatory myofibroblastic tumors (IMT), and 11 follicular dendritic cell sarcomas using three antibody clones: D5F3, 5A4, and ALK1.
Read More »Molecular pathology selected abstracts
March 2019—Using circulating cell-free fetal DNA to test for monogenic disorders: Screening for fetal chromosomal abnormalities can be performed by noninvasive methods in which fetal cell-free DNA (cfDNA) circulating in the maternal blood is isolated and analyzed. However, the standard of care for screening for monogenic diseases remains population-based carrier screening—testing the parents for their carrier status of deleterious genes.
Read More »Digital pathology matchmaking: people, pixels
February 2019—Digital pathology is many things. One thing it’s not is a one-night stand. As laboratories contemplate using digital pathology for primary diagnosis in the wake of the FDA’s approval nearly two years ago, it’s become abundantly clear that while digital pathology might seem to promise easy pleasure, it’s actually as complicated as keeping multiple spouses happy. Think Jacob, Rachel, and Leah. Think “Big Love.”
Read More »HbA1c platforms studied for lipemia interference
February 2019—A forgotten creditor. A poor relation. An envious rival. In the theater, one of these characters often emerges from the woodwork, ready to supply a plot twist just when the protagonist is riding highest.
Read More »Apocrine breast cancer, ESR1 mutations at center of tumor board review
February 2019—Two breast cases—one of apocrine carcinoma and androgen receptor overexpression and another of metastatic ER-positive cancer and an ESR1 mutation—were the focus of a molecular oncology tumor board session at CAP18.
Read More »Conference shines light on latest in mass spec
February 2019—Mass spectrometry has been proved to be an essential and powerful platform for clinical diagnostics. The value it adds to health care has increased steadily in the past decade.
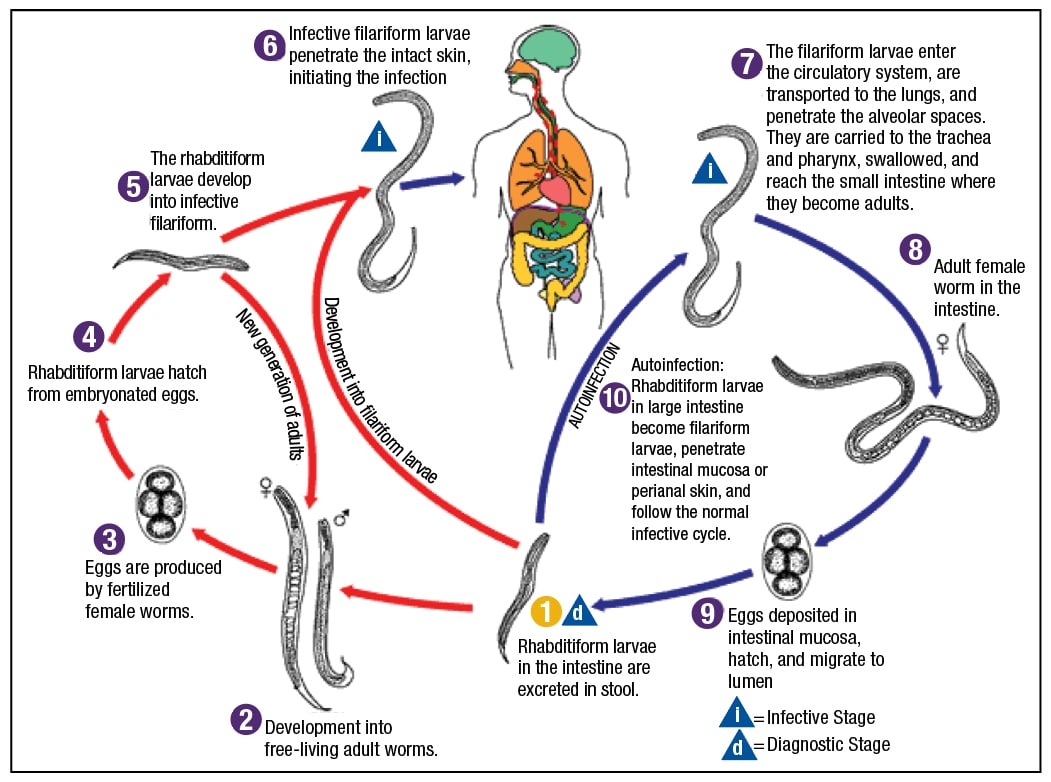
Read More »Know them when you see them: parasites in tissue
February 2019—In one case, Dr. Ribes found herself intrigued by structures in a patient’s urine cytology specimen. A 36-year-old man from the Republic of Congo had developed dysuria, increased urinary frequency, and terminal hematuria. What they found in the Pap stain in cytology, she said, were large purple structures with the knob at the end.
Read More »AMP case report: Diagnostic pitfalls of testing rare molecular aberrations in lung adenocarcinomas
February 2019—Lung cancer is the second most commonly diagnosed malignancy and results in the most cancer-related deaths each year in the United States, but actionable aberrations in EGFR, ALK, ROS1, and other oncogenes are improving outcomes for a subset of patients.
Read More »Not a fan of training new hires? Lab finds a fix
February 2019—When Tania Hong, BS, MT, joined the University of Vermont Medical Center five years ago, as network director of operations for pathology and laboratory medicine, she interviewed all of the supervisors in her laboratory. She asked what they saw as their biggest challenges.
Read More »AP-LIS vendors on AI, practice complexity, the cloud
February 2019—Practice consolidation and complexity, Web-based and cloud computing, and artificial intelligence. That and more is what writer Valerie Neff Newitt asked six AP-LIS companies about recently. Here is what they had to say. The profiles of their AP information systems are in the Anatomic pathology product guide, along with those of 16 other companies.
Read More »Standard provides labs with predefined LOINC codes for IVD tests
February 2019—Logical Observation Identifiers Names and Codes, or LOINC, is a universal code system for identifying laboratory and clinical observations. LOINC provides unique numeric codes that identify the type of in vitro diagnostic test with defined attributes including component, property, time, system, scale, and method.
Read More »No diagnostics, no stopping antibiotic misuse
February 2017—In a context where the lack of drugs against resistant bacterial pathogens will continue and antimicrobial prescriptions are highly complex, effective antibiotic stewardship programs are strongly needed. In the U.S., the CDC and Centers for Medicare and Medicaid Services highly recommend, while the Joint Commission requires, that all hospitals and nursing care centers have antimicrobial stewardship programs, effective January 2017.
Read More »Put It on the Board
Hologic assay is first FDA-cleared test to detect M. genitalium
February 2019—The Food and Drug Administration granted clearance for Hologic’s Aptima Mycoplasma genitalium assay. This is the first test the FDA has authorized for M. genitalium detection. The FDA reviewed data from a clinical study that included testing of 11,774 samples. The FDA says the study showed that the Aptima assay correctly identified M. genitalium in approximately 90 percent of vaginal, male urethral, male urine, and penile samples. It correctly identified M. genitalium in female urine and endocervical samples 77.8 percent of the time and 81.5 percent of the time, respectively. Vaginal swabs are the preferred sample type owing to better clinical performance. Alternative sample types, such as urine, can be used if vaginal swabs are not available. In addition, the study showed that the test correctly identified samples that did not have M. genitalium present 97.8 to 99.6 percent of the time. The FDA reviewed the Aptima M. genitalium assay through the de novo premarket pathway.
Q&A column
Q. When performing a manual differential that contains immature cells, such as metamyelocytes and myelocytes, do you report an absolute count on all of the individual cells in the myelocytic line, or do you group them together and calculate one ANC? What about lymphocytes and reactive lymphocytes? Read answer. Q. Why and in what employment screening settings is the two-step skin test for Mycobacterium tuberculosis recommended? Read answer.
Read More »From the President’s Desk: Giving group practices what they need most
February 2019—In last month’s column, we talked about practice engagement as an umbrella term for laboratory medical direction and practice management that builds strong relationships within and beyond the laboratory. The CAP Practice Management Committee has been taking the lead on this, but it cuts across multiple domains; the conundrum, as always, is the complexity of what we do. Practice management tools designed for other settings cannot meet our needs because we must address economy, efficiency, effectiveness, and collegiality concerns specific to pathology. But then, affinity for complexity is how we landed here in the first place, so that plays to our strengths.
Read More »Clinical pathology selected abstracts
February 2019—Impact of commercial laboratory testing on a care delivery system: Care delivery systems have become increasingly fragmented and complex, which impacts patient care. The amount of health care data generated has also created a problem by reducing the time devoted to direct patient care.
Read More »Anatomic pathology selected abstracts
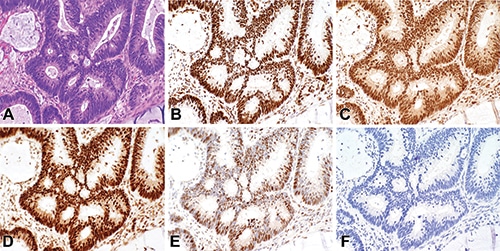
February 2019—Diagnostic algorithmic proposal based on IHC evaluation of invasive endocervical adenocarcinomas: The International Endocervical Adenocarcinoma Criteria and Classification was developed to separate endocervical adenocarcinomas into two main categories based on morphology: human papilloma virus-associated (HPVA) and nonhuman papilloma virus-associated adenocarcinomas.
Read More »Molecular pathology selected abstracts
February 2019—Evaluation of mutational processes and somatic driver mutations in cancer exomes: As next-generation sequencing-based tumor profiling gains popularity, multiple informative variables other than single-gene alterations will continue to be added to clinical reports.
Read More »Newsbytes
February 2019—Digital pathology RFPs: from the questions to selections: To those who request the information and those who supply the information, requests for proposal, better known as RFPs, can be groanworthy. Yet laboratories planning to purchase a digital pathology system for clinical use should seriously consider going through the painstaking process, even if their institutions don’t require it, says Liron Pantanowitz, MD, vice chair of pathology informatics at the University of Pittsburgh Medical Center.
Read More »Taking measure of cholangiocarcinoma
January 2019—Ten years ago, says Manhal Izzy, MD, the approach might have seemed quixotic: performing liver transplants in patients with early intrahepatic cholangiocarcinoma. Even today, it’s hardly standard of care. Nevertheless, it has moved well beyond the impossible dream category. Medicine advances, and practices change. There’s nothing unusual about that. But Dr. Izzy, assistant professor of medicine, Vanderbilt University School of Medicine, and transplant hepatologist, Vanderbilt University Medical Center, goes out of his way to credit the wisdom and work of those in hepatology and oncology who have been pushing forward curative approaches for cholangiocarcinoma.
Read More »Navigating the quandaries of coagulation testing
January 2019—Naming the things about coagulation testing that most perplex clinicians isn’t easy for Michael Laposata, MD, PhD. But there’s a good reason for that: He finds confusion to be pervasive.
Read More »At-home testing for heart failure, transplant patients: Can it work?
January 2019—Medicare hospital readmission rates are down under the Centers for Medicare and Medicaid Services readmissions reduction program, though hospitals are still paying millions in penalties, in part because new conditions are included in the calculations.
Read More »Drug-susceptibility testing for TB: poised to take a turn?
January 2019—In a large international study, whole genome sequencing with next-generation sequencing technology has proved its ability to accurately assess susceptibility of Mycobacterium tuberculosis isolates to four first-line drugs.
Read More »Parasites in tissue: how to identify the structures
January 2019—Pathologists who aren’t microbiologists can provide a diagnosis of parasitic disease if they take into account parasite life cycles and tissue tropisms. Julie A. Ribes, MD, PhD, made that key point in cases she presented in her CAP18 session, “Update on Invasive Parasitic Infections for Surgical Pathologists.” Dr. Ribes added learning material to most of the cases, she said, but the cases come from parasites she has seen and known in her own professional life.
Read More »AMP case report: Identification by NGS of a diagnostic and theranostic mutation in a high-grade sarcoma of the humerus
January 2019—A 75-year-old woman with history of melanoma localized to the right forearm and status post excision six years prior presented with a two-month history of continuous left shoulder pain. She was managed initially with physical therapy and hydrocodone with no effect. Initial workup at an outside institution included an x-ray of the left shoulder that showed a destructive lytic lesion involving the proximal aspect of the left humerus associated with a pathologic fracture.
Read More »Cytopathology in focus: How can a lab ensure individual competence?
January 2019—It is happening again: CAP members and cytotechnologists are asking about regulatory requirements for re-integrating into cytopathology after a period of practice latency. That is good news because it indicates that they are interested in practicing at a time when the cytopathology community can use skilled professionals. The past decade has seen a shrinking volume of Pap tests and a concomitant decline in the number of practicing cytologists, which has created new job opportunities for those with cytopathology skills.
Read More »Cytopathology in focus: Serous fluid cytology and the international system
January 2019—Just when you thought you were done implementing a new terminology for cytology, another one pops up. Is it possible there are sites that have not yet been standardized? Unbelievably, the most common nongynecologic cytology specimen, body fluids, remains a Wild West for terminology.
Read More »Cytopathology in focus: Next-generation cytotechnology—new cytotechnologist roles
January 2019—The evolution of minimally invasive techniques and new diagnostic modalities have placed new demands on medical laboratories. Cytotechnologists find themselves uniquely poised to take on these new responsibilities, using their morphologic and analytical skills. In this article, I summarize potential roles for cytotechnologists that go beyond screening cytology slides to add value and improve quality in the clinical laboratory.
Read More »Cytopathology in focus: Three special reports capture a field in transition
January 2019—In the September/October 2018 issue of the Journal of the American Society of Cytopathology are three special reports from the American Society of Cytopathology/American Society for Clinical Pathology workgroup on current practices and future perspectives for the field of cytotechnology.
Read More »Cytopathology in focus: ABPath CertLink pilot open to all
January 2019—The American Board of Pathology subspecialty certification examination in cytopathology is given each fall at the ABPath office in Tampa, Fla. Cytopathology has the largest number of examinees of the 11 pathology subspecialty examinations.
Read More »Put It on the Board
FDA clears 2 of 3
ePlex blood culture ID panels:
January 2019—GenMark Diagnostics announced in December that it received FDA market clearance for its ePlex Blood Culture Identification Gram-Positive (BCID-GP) and Fungal Pathogen (BCID-FP) panels. GenMark’s third panel, ePlex Blood Culture Identification Gram-Negative (BCID-GN), was submitted to the FDA in September 2018 and is still under review. The fungal pathogens panel has broad coverage and includes many resistant and emerging strains, among them Candida auris, GenMark said in its statement. The company says that by coupling BCID panels with the ePlex Templated Comments software module, hospitals can enable immediate intervention linked to a diagnostic result and improve the effectiveness of antimicrobial stewardship initiatives.
12 assays for Atellica Solution:
Siemens Healthineers achieved 12 pre-market approvals from the FDA for its Atellica Solution infectious disease and oncology testing menu.
Q&A column
Q. Which gynecological slides can cytotechnologists report? Read answer. Q. Is it recommended that a hospital streamline its reference ranges for point-of-care and main laboratory tests? Read answer.
Read More »Newsbytes
January 2019—Virtual tumor board platforms: a game changer for cancer case review: If Suneal Jannapurredy, MD, had been able to read the patient’s outside radiology report prior to breast tumor board, he would have re-examined the gross specimen to determine whether, as the other providers were now telling him, there was a second area of focus he hadn’t included in his presentation.
Read More »From the President’s Desk: Practice engagement resources for all
January 2019—Excellence in the laboratory can have a powerful impact on the culture of our institutions because we come into contact with so many and so much. Mostly, we just need to do complex things extremely well and make it look easy. In other words, practice pathology. The more than 50 pathologists and numerous laboratory professionals in my group practice, Delta Pathology, serve nearly 100 institutions across Louisiana and Mississippi.
Read More »Clinical pathology selected abstracts
January 2019—RH genotype matching for transfusion support in sickle cell disease: In patients with sickle cell disease, Rh alloimmunization remains a challenge, despite the transfusion of serologic Rh C, E, and K antigen-matched red cells.
Read More »Anatomic pathology selected abstracts
January 2019—Distinguishing patients with double somatic mismatch-repair mutations from those with Lynch syndrome: Lynch syndrome is the most common form of hereditary colon cancer. Germline mutations in the mismatch-repair (MMR) genes MLH1, MSH2 (EPCAM), MSH6, and PMS2, followed by a second hit to the remaining allele, lead to cancer development.
Read More »Molecular pathology selected abstracts
Editors: Donna E. Hansel, MD, PhD, chief, Division of Anatomic Pathology, and professor, Department of Pathology, University of California, San Diego; James Solomon, MD, PhD, resident, Department of Pathology, UCSD; Richard Wong, MD, PhD, molecular pathology fellow, Department of Pathology, UCSD; and Sounak Gupta, MBBS, PhD, molecular pathology fellow, Memorial Sloan Kettering Cancer Center, New York. Development of brain circuits ...
Read More »Daniel J. Hanson, MD, 1928–2018
January 2019—Daniel J. Hanson, MD, a member of the CAP Board of Governors from 1993 to 1999, died on Nov. 2, 2018. Dr. Hanson was president and medical director of Pathology Laboratories in Toledo, Ohio, until he retired in 2005. He was also clinical professor of pathology emeritus at the University of Toledo College of Medicine.
Read More »Rapid PCR rules as labs ready flu arsenal
December 2018—With the memory of the 2017–2018 “high-severity” influenza season fresh in mind—49 million cases, 960,000 hospitalizations, a marginally effective vaccine, 79,000 deaths—clinical laboratories have been bracing for the customary annual surge in emergency room, outpatient clinic, and physician office influenza test orders. Although flu admissions have been rising somewhat, it is too soon to know how the season will play out, but laboratories are hoping for a season closer to average. Avoiding a repeat of last year’s travails—lengthy turnaround times, supply shortages, and the need to triage patients for testing—is a must, many laboratory directors say. “We had difficulty keeping up with last year’s demand. It was extremely time-consuming,” says Mary Kay O’Connor, national laboratory director at Summit Health Management, the management arm of the Summit Medical Group, an 800-provider practice on the East Coast.
Read More »Multiplex for allergy dx: powerful, but it has its place
December 2018—In allergy testing, microarray technology offers speed and the benefit of smaller sample volumes, but it has a lower sensitivity and is unable to detect IgE antibodies of all specificities in a given extract unless all allergens are on the chip. For routine use, singleplex assays are here to stay.
Read More »Could CGM dethrone HbA1c for office-based diabetes care?
December 2018—A glucose sensor the size of a quarter placed on the body and a sensor filament inserted under the skin could potentially disrupt traditional diabetes care with its continuous monitoring of glucose almost 300 times a day. Blood glucose can fluctuate widely during the day even in completely healthy people, said David Sacks, MB, ChB, in an interview with CAP TODAY.
Read More »Program zeroes in on histology, digital scan connection
December 2018—Quality in histology is at the heart of successful whole slide imaging, and a new program that rolls out in January will provide laboratories the aid they may need as they bring whole slide imaging on board.
Read More »Drug overdose deaths and toxicology tests: Let’s talk
December 2018—Drug overdose deaths in the United States continue to rise, and recently many of these deaths have been attributed to opioids, including fentanyl, fentanyl analogs, and other opioid receptor agonists. The rise in drug overdoses and drug-related deaths, and the devastating effects of the opioid crisis, highlight the need for communication and coordination among forensic pathologists, hospital clinicians, and laboratorians.
Read More »Higher pay for fibrinolysins interpretation in ’19 fee schedule
December 2018—The CMS finalized its 2019 Medicare physician fee schedule and its response to the CAP’s recommendations to raise payment for fibrinolysins interpretation and reporting and to forgo a proposed decrease to the physician work value for blood smear interpretation. The Centers for Medicare and Medicaid Services on Nov. 1 published the 2019 physician fee schedule. Services on the physician fee schedule are composed of three relative value units designated by the CMS: physician work, practice expense, and malpractice liability RVUs. Each RVU is separately valued and summed to equal the total RVU for each physician service on the fee schedule. The CAP advocates for the appropriate valuation of pathology services through its representation on the advisory committee of the AMA/Specialty Society Relative Value Scale Update Committee, known as RUC.
Read More »Urinalysis instrumentation: All eyes on standardized, scalable, integrated solutions
December 2018—On page 36 begins our urinalysis instrumentation product guide. The companies whose analyzers are profiled talked to CAP TODAY writer Valerie Neff Newitt
Read More »AMP case report: Discordant IHC/PCR test results for mismatch repair status in colorectal adenocarcinoma
December 2018—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Duke University Medical Center. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Read More »Put It on the Board
Pathologists and population health—
first steps
December 2018—Pathologists and population health—first steps: Pathologists who want to become involved in population health initiatives can take five main steps, say pathologists and laboratory leaders interviewed for an article published online last month in Archives of Pathology & Laboratory Medicine. In “The role of the pathologist in population health,” the authors report on the interviews they conducted and their review of the literature to answer several questions, among them whether pathologists in both large settings and smaller community-based settings can engage in population health (yes), and whether pathologists are in a position to analyze data for population health (“The data are there,” they say, “but getting to the data—and providing meaning out of it—is the hard part”). One of the first steps to becoming involved in any type of population health management activities, the authors write, is to understand the philosophy of the institution’s CEO and senior management.
Q&A column
Q. Can you explain further the revised CAP checklist requirement COM.40850 “LDT and Class I ASR Reporting,” which says to describe the method and performance characteristics in test reports unless the information is available to the clinician in an equivalent format? Read answer. Q. Can we see reactive lymphocytes in the pediatric population (under age two), and can we report them? Read answer.
Read More »Newsbytes
In molecular testing labs, gaps between actual and desirable LIS capabilities
December 2018—Flashback to 2013: Alexis B. Carter, MD, then director of pathology informatics at Emory University Hospital, was contemplating whether other pathology labs nationwide were facing the same challenges managing molecular testing data as she and her colleagues. So she decided to find out. Dr. Carter conducted a survey, and the responses confirmed her suspicions: Most laboratory information systems fall short in providing the infrastructure for complex molecular and genomic testing.
From the President’s Desk: Embracing our future at CAP18
December 2018—The CAP Curriculum Committee chaired by Sarah M. Bean, MD, follows a competency-based model to build an annual meeting program that is practical, prescient, and diverse. The committee is balanced demographically, experientially, and scientifically. Whatever the topic, there is someone who can speak to it.
Read More »Clinical pathology selected abstracts
Prostate cancer screening with PSA test: systematic review and meta-analysis
December 2018—Prostate cancer is the second most common cancer and the fifth leading cause of cancer-associated mortality among men worldwide. The use of serum prostate-specific antigen (PSA) to screen for prostate cancer is intended to detect the cancer at an early stage to reduce overall and disease-specific mortality. However, evidence that PSA screening for prostate cancer saves lives is somewhat lacking. Read More »Anatomic pathology selected abstracts
December 2018—Applying deep convolutional neural networks to diagnostic breast biopsies: The breast stromal microenvironment is a pivotal factor in breast cancer development, growth, and metastases. Although pathologists often detect morphologic changes in stroma by light microscopy, visual classification of such changes is subjective and nonquantitative, limiting its diagnostic utility.
Read More »Molecular pathology selected abstracts
December 2018—Profiling of chromatin-accessibility landscape of primary cancers: The chromatin-accessibility profiles generated in this study by The Cancer Genome Atlas represent the largest pan-cancer effort to characterize the regulatory landscape of human cancers. The study primarily used an assay for transposase-accessible chromatin using sequencing (ATAC-seq).
Read More »Time now for tumor mutational burden?
November 2018—Like a piece of so-called sticky music, cutoff numbers can persist in physicians’ minds outside of any real clinical value and, in the process, leave their laboratory colleagues mildly befuddled (not to mention searching for more useful cutoffs). Such a jingle is creeping into tumor mutational burden. Lauren Ritterhouse, MD, PhD.
Read More »New momentum for harmonizing lab results
November 2018—In The Music Man, the four members of the local school board, notorious for their squabbling, are assigned to investigate the credentials of “professor” Harold Hill, a charming con man who has come to town to sell band uniforms and instruments.
Read More »Biotin interference: answering questions, reducing the risk
November 2018—Biotin use is not rare, and don’t count on it being listed in the patient’s electronic medical record. Those are some of the findings of a Mayo Clinic study published recently in Clinical Biochemistry.
Read More »Component IgE testing offers food for thought
November 2018—Food component testing offers improved specificity for distinguishing IgE sensitized from truly allergic patients, and the menu for allergen components may soon expand.
Read More »AMP case report: Detection of concurrent hematologic malignancies in solid tumor NGS testing may cause false-positive results
November 2018—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Weill Cornell Medicine.
Read More »Inside the Color Atlas of Mycology: Candida famata
November 2018—Color Atlas of Mycology: An Illustrated Field Guide Based on Proficiency Testing is a new book from CAP Press, released in October. It is designed to help in identifying fungi using the most recent taxonomic classifications. In it is more than 15 years of proficiency testing data to highlight diagnostic clusters of incorrect identifications and address conceptual classification issues. Following is an excerpt from the section on yeast.
Read More »Laboratory information system vendors on where their focus is
November 2018—CAP TODAY’s lineup of laboratory information systems begins on page 41. Here, six of the 30 companies that have LISs listed in the guide tell us what you, our readers, want from your LIS and what they want you to know about them.
Read More »Hematology roundtable: rules, reference ranges, POC testing
November 2018—Reference intervals, point-of-care testing, the use of rules for efficiency, and the display of results in patient records. That and more is what a panel of experts weighed in on when CAP TODAY publisher Bob McGonnagle assembled them in September to talk about hematology instrumentation. What they told us follows.
Read More »On Roche m 511 analyzer, ‘everything is done from the slide’
November 2018—Roche Diagnostics will soon launch its m 511 analyzer for hematology laboratories. Krista Curcio, Roche technical marketing manager, hematology, told us, in a recent conversation with CAP TODAY publisher Bob McGonnagle, how and why the new instrument is different. “We’re turning it upside down and going a different way,” she said of the m 511. Here is more on the instrument Roche will launch before year’s end.
Read More »Put It on the Board
Quest acquires PhenoPath
November 2018—Quest Diagnostics has acquired PhenoPath Laboratories, which provides immunophenotyping, hematopathology, and molecular pathology services. The PhenoPath business, in Seattle, will operate as part of AmeriPath, a wholly owned business of Quest. Steve Rusckowski, Quest chairman, president, and CEO, said in a statement: “PhenoPath has a strong record of innovation and provides several capabilities that complement and extend our own, particularly in pathology and molecular oncology. It also deepens our presence in the Pacific Northwest.” PhenoPath founder Allen Gown, MD, tells CAP TODAY that continued consolidation in the laboratory industry and insurance reimbursement challenges have posed significant risks to PhenoPath’s future growth. “In Quest/AmeriPath,” he says, “we found an organization that realized not only the excellence of PhenoPath’s past and present but also the extraordinary future that, with their assistance, we can have.” Dr. Gown founded PhenoPath in 1998.
Q&A column
Q. Is anticoagulant adjustment in citrate tubes necessary when a patient’s hematocrit is less than 20 percent? Read answer. Q. What is the substitute test for HbA1c for a patient with homozygous variant hemoglobin? Is a fructosamine and/or glycated albumin test appropriate? Read answer.
Read More »Newsbytes
How Henry Ford core lab uses bottom-up communication November 2018—When Henry Ford Health System started planning its core laboratory’s automation line four years ago, aware that it needed to take Lean to the next level, it enlisted frontline laboratory employees in a development process that used the strategies of Hoshin Kanri and kaizen. Read more.
Read More »From the President’s Desk: Advocacy—Whose job is it anyway?
November 2018—Last month, we talked about how the CAP Laboratory Accreditation Program employs mentorship and perspective to move our specialty forward. I’d like to talk this month about how we apply those tools in our advocacy program.
Read More »Clinical pathology selected abstracts
Preparing for passage of regulatory requirements for laboratory-developed tests: November 2018—The FDA has raised concerns, in recent years, about several high-risk laboratory-developed tests (LDTs), including a concern that patients may undergo unnecessary treatment or delay or forego treatment due to the inaccuracy of such tests. Other agencies have also challenged the validity, accuracy, oversight, and safety of LDTs, a subset of IVDs that are intended for clinical use and designed, manufactured, and used within a single laboratory.
Read More »Anatomic pathology selected abstracts
November 2018—HER2: a pan-cancer event highly enriched in AR-driven breast tumors: Approximately one in five breast cancers is driven by amplification and overexpression of the HER2 receptor kinase, and HER2 enriched is one of four major transcriptional subtypes of breast cancer. The authors conducted a study to understand the genomics of HER2 amplification independent of subtype, as well as the underlying drivers and biology of HER2-enriched (HER2E) tumors.
Read More »Molecular pathology selected abstracts
November 2018—Common genetic variants contribute to risk of rare severe neurodevelopmental disorders: The traditional paradigm broadly classifies genetic diseases into rare disorders caused by a single gene variant and common disorders caused by complex interplay among multiple genes. However, recent research has shown that penetrance and disease phenotype, even in disorders thought to be monogenic, are affected by common genetic variation.
Read More »Letters: Ph-like ALL
November 2018—In the October issue of CAP TODAY, Karen Titus shared with us a story titled, “Fresh incentive to look for Ph-like ALL.” She spoke with key players in the discovery of BCR-ABL1-like B-lymphoblastic leukemia/lymphoma (B-ALL) (or “Ph-like” ALL), which is now a provisional entity in the 2016 revised fourth edition of the WHO. A key takeaway from the article ...
Read More »AMP case report: October 2018 test yourself answers
In the October 2018 issue was a case report (page 70), “NGS in the diagnosis of RASopathies in histologically uninformative skin biopsy samples,” written by members of the Association for Molecular Pathology. Here are answers (in bold) to the three “test yourself ” questions that followed that case report. 1. Which of the following is the most commonly mutated gene in ...
Read More »Fresh incentive to look for Ph-like ALL
October 2018—Cheryl Willman, MD, could hardly believe her eyes. She and her colleagues at the University of New Mexico, working with collaborators from across the U.S. in the NCI TARGET Project, had submitted 100 cases of high-risk pediatric acute lymphoblastic leukemia to British Columbia’s Cancer Agency for RNA sequencing to figure out why patients were doing so poorly, despite treatment with intensive chemotherapy.
Read More »Thyroid during pregnancy: how it changes, how to test
October 2018—How pregnancy affects normal thyroid function and thyroid function tests, and screening for thyroid disease during pregnancy, were the focus of a session at this year’s AACC annual meeting.
Read More »New requirements for use and storage of liquid nitrogen, dry ice
October 2018—Laboratory personnel safety is at the center of two new requirements and a revised requirement in the latest edition of the CAP accreditation program checklists, released in August.
Read More »Revenue cycle services: can they quell billing woes?
October 2018—Envision a long stretch of roadblocks of all shapes and sizes, staffed by unsympathetic and ever-changing guards, with new roadblocks springing up all the time, and you’ll probably have a fix on how most laboratories have viewed billing, going back decades.
Read More »New accreditation program checklist section: Imaging mass spec scores its own quality standards
October 2018—It happened for next-generation sequencing. It was an important step for in vivo microscopy. And now it’s taking place with imaging mass spectrometry. The milestone: development and adoption of a set of specialized checklist requirements for laboratories that want CAP accreditation. Imaging mass spectrometry, an adjunct methodology to help pathologists analyze areas of interest in tissue specimens, is, at this point, used in a small number of research laboratories in the U.S., says CAP Checklists Committee member Christopher M. Lehman, MD, clinical professor of pathology, University of Utah College of Medicine, and medical director of the University of Utah Hospital Laboratory.
Read More »New Color Atlas aids in identifying fungal species
October 2018—CAP Press released in October the Color Atlas of Mycology, by Gordon Love, MD, D(ABMM), and Julie Ribes, MD, PhD. Its 388 pages hold more than 800 tables and images, with identifications verified by DNA sequencing (for images post-2009). Here, in an exchange with CAP TODAY, Dr. Love explains how this atlas stands apart from others in the Color Atlas series and from others on the market.
Read More »Automated molecular platforms: 3 companies on what’s new and next
October 2018—CAP TODAY’s updated guide to the automated molecular platform market begins on page 45. Thirty-four platforms are profiled, with one new one: Hologic’s Panther Fusion. Writer Valerie Neff Newitt talked with three of the 20 companies about what they introduced this year, what’s to come, and more. “This is a dynamic and competitive industry. We are always asked to go faster, and that is what we are trying to do in terms of development,” says Michelle Tabb, PhD, chief scientific officer, DiaSorin Molecular. Others seem to be doing the same.
Read More »AMP case report: NGS in the diagnosis of RAS opathies in histologically uninformative skin biopsy samples
October 2018—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from the University of Washington, Seattle. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Read More »Shorts on Standards—Establishing cutoffs, reference ranges for biofluid biomarkers in Alzheimer’s disease: Reference materials and reference measurement procedures
October 2018—The newly developed National Institute on Aging and Alzheimer’s Association (NIA-AA) research framework uses a biological definition of Alzheimer’s disease.1 This framework has increased focus on biofluid biomarkers, especially because the measurement of cerebrospinal fluid amyloid beta peptide 42 (Aβ42) (or Aβ 42/40 ratio), phosphorylated tau protein (p-tau), and total tau proteins (T-tau) are included in the definition.1 The field of AD biofluid biomarkers is rapidly evolving. For example, CSF neurofilament light (NfL) is associated with AD neurodegeneration and may be a better CSF marker compared with T-tau.
Read More »Put It on the Board
Therascreen EGFR RGQ PCR kit approved as companion diagnostic for Vizimpro
October 2018—The FDA has approved a PMA supplement expanding the labeling claim of the Qiagen Therascreen EGFR RGQ PCR kit to allow its use as a companion diagnostic with Pfizer’s Vizimpro (dacomitinib). Vizimpro is for first-line treatment of patients with non-small cell lung cancer with EGFR exon 19 deletions or an exon 21 L858R mutation. The Therascreen EGFR RGQ PCR kit is now approved as a companion diagnostic to guide the use of three FDA-approved therapies, including also Gilotrif (afatinib) from Boehringer Ingelheim and Iressa (gefitinib) from AstraZeneca. It is registered in more than 40 countries. This was a project governed under an agreement between Qiagen and Pfizer.
Philips introduces computational pathology software for tumor detection
Royal Philips announced in September the latest release of TissueMark, which the company says now supports region of interest detection for the majority of molecular testing.
Q&A column
Q. How can one wisely apply GATA3 immunohistochemistry as a useful tumor marker in diagnostic surgical pathology? Read answer.
Read More »Newsbytes
Innovation labs: different means to the same end October 2018—What’s the best way for a hospital to kill a health care improvement-related idea? Some say (tongue in cheek) send it to committees, meetings, suggestion boxes, or the like. Read more.
Read More »From the President’s Desk—CAP accreditation: perspective-taking 101
October 2018—I grew up in the CAP as a volunteer in the Laboratory Accreditation Program. It’s a good place to dive in. Many of us do what I did—work our way through many volunteer opportunities over 30 years or more because each was so interesting. There are an amazing number of ways a person can approach challenges in a laboratory; more amazing is how many of the approaches will work. Partly that’s because the learning cuts both ways—I’ve learned as much when we were inspecting another laboratory as when my laboratory was being inspected.
Read More »Clinical pathology selected abstracts
RT-PCR detection of B. microti parasites using BMN antigens as amplification targets October 2018—Babesia microti infection, which is transmitted through the bite of an infected tick, is a growing health concern and continues to be a threat to the blood supply, with 22 states having reported cases of babesiosis in 2014. While most healthy adults with Babesia infection are asymptomatic or present with mild symptoms, including fever, fatigue, and anemia, babesiosis can be severe or fatal in neonates, the elderly, and immunosuppressed individuals.
Read More »Anatomic pathology selected abstracts
Analysis of ZC3H7B-BCOR high-grade endometrial stromal sarcomas October 2018—High-grade endometrial stromal sarcoma likely encompasses underrecognized tumors harboring genetic abnormalities besides YWHAE–NUTM2 fusion. Triggered by three initial endometrial stromal sarcomas with ZC3H7B–BCOR fusion characterized by high-grade morphology and aggressive clinical behavior, the authors investigated the clinicopathologic features of this genetic subset by expanding the analysis to 17 such tumors. All of the tumors occurred in women who were a median age of 54 (range, 28–71) years.
Read More »Molecular pathology selected abstracts
Cell-free DNA tumor mutational burden predicts efficacy of immune checkpoint inhibitors October 2018—Immune checkpoint inhibitors have emerged as a potent class of therapy for a variety of malignancies. The biologic rationale for these drugs is that somatic mutations, not necessarily in cancer driver genes, may accumulate in tumor cells, resulting in amino acid changes that create neoantigens (epitopes not present in normal cells during maturation of the immune system).
Read More »Letters
AMH immunoassays October 2018—In the article “Satisfaction high with new automated AMH assays” (June 2018), the focus seems to be on the presumed advantages of the Roche Elecsys and Beckman Coulter Access automated anti-müllerian hormone assays over manual AMH assays. The article reports that the main advantages of the automated platforms are less variability in AMH measurements, greater assay turnaround time, and greater assay cost-effectiveness.
Read More »Molecular ‘bucket list’ for renal cancer
September 2018—Leo Tolstoy is not listed as a coauthor on the most recent iteration of The Cancer Genome Atlas on renal cell carcinoma, which focuses on molecular characterization of RCC.
Read More »Hemophilia drug interferes with APTT-based assays
September 2018—When a miracle drug comes along that is predicted to cause havoc in the laboratory, the drug could well seem like a double-edged sword. In the case of emicizumab (Genentech’s Hemlibra), for patients with hemophilia A, the mix of both benefits and drawbacks is likely to settle in for the long term.
Read More »Forward march on commercial NAAT for M. genitalium
September 2018—About five years ago, when William M. Geisler, MD, MPH, was still focusing his research at the University of Alabama at Birmingham on chlamydia, Mycoplasma genitalium carried a lower profile as a cause of sexually transmitted infection.
Read More »For autopsy service, new requirements in AP checklist plus nine new requirements for forensic autopsies
September 2018—Quality management, communication, and consent are among the issues addressed in new and revised requirements in the autopsy pathology section of the latest edition of the CAP accreditation program anatomic pathology checklist.
Read More »From the President’s Desk: Policies to protect and preserve
September 2018—CAP leadership presents a gamut of responsibilities, including the enforcement of policies adopted to protect members and staff. What I am about to discuss is relevant to all organizations and work settings. As you read on, I hope you will reflect on how tolerance for inappropriate behavior could have an impact on your own workplace and what steps you can take to protect yourself, your colleagues, and by extension your patients.
Read More »Urine drug testing debate: How best to test compliance and manage opioid crisis
September 2018—Qualitative or quantitative testing. Hydrolyze or don’t hydrolyze. Use or don’t use standard cutoffs. These and other decisions in toxicology testing have taken on new urgency amid the opioid crisis, which is driving laboratories to change test methods to assess prescription drug compliance and illicit drug use.
Read More »‘We wanted to be the best we could possibly be’: CAP ISO 15189-accredited labs on the difference it makes
September 2018—Ten years ago, Richard J. Zarbo, MD, was feeling pretty proud of his laboratory. As system chairman of pathology and laboratory medicine at Detroit-based Henry Ford Health System, over the previous few years he’d seen his team rigorously implement Lean practices, practices that had paid off in greater safety and efficiency. “Setting the bar higher was important because that’s the culture here,” he says. “This is what we do.”
Read More »Next step? The switch from stool culture to PCR
September 2018—The advantages of moving from stool culture to a molecular platform are many: faster time to results, more accurate pathogen identification, a savings of space and staff time. For Jose Alexander, MD, D(ABMM), SM, MB(ASCP), and colleagues at Florida Hospital Orlando, another plus is being able to adhere to the Infectious Diseases Society of America guideline suggestion that labs use a diagnostic approach that can distinguish O157 from non-O157 E. coli and Shiga toxin 1 from Shiga toxin 2 E. coli.
Read More »Xifin CEO: Time to tune up negotiations with payers
September 2018—The second round of PAMA data collection is coming in 2019 and it’s critical to get it right, said Lâle White, CEO of Xifin, in a presentation in May at the Executive War College. If it’s not right, she warned, laboratories could see cuts that are more severe than those already seen.
Read More »Put It on the Board
Broad-based molecular testing for NSCLC September 2018—A recently published study on broad-based genomic sequencing and survival among patients with advanced non-small cell lung cancer in the community oncology setting should not lead to the conclusion that such sequencing should be avoided in nonsquamous NSCLC, say Paul A. Bunn Jr., MD, and Dara L. Aisner, MD, PhD, of the University of Colorado Denver, Aurora. Dr. Bunn, of the Department of Medical Oncology, and Dr. Aisner, of the Department of Pathology, in an editorial published Aug. 7 in JAMA, caution readers about the study published in the same issue, which found that broad-based sequencing (more than 30 cancer genes) directly informed treatment in a minority of patients and was not independently associated with better survival. The study of 5,688 patients with advanced NSCLC was based on data acquired through abstraction and aggregation of information from the electronic medical record from 191 U.S. community oncology practices.
Read More »Q&A column
Q. Is there expert advice or standard practice for releasing preliminary critical values for patients to the LIS pending subsequent technologist or technician verification and documentation? Read answer. Q. We hope to validate a procedure for the fixation, decalcification, and staining of bone marrow specimens but we will not be able to access fresh marrow specimens for our decalcification validation. Can you recommend an alternative tissue to validate the preservation of tissue morphology and antigenicity after decalcification? Read answer.
Read More »Clinical pathology selected abstracts
Outcomes of an audit of repeat lab testing at an academic medical center
September 2018—Overutilization of laboratory tests increases health care costs and may lead to false-positive test results and ambiguous findings. Unnecessary testing can result from a single order from a provider, an automated function in an order set, or a combination of the two.
Anatomic pathology selected abstracts
Thymoma: a clinicopathological correlation of surgical resection cases
September 2018—The authors presented 1,470 surgical resections for thymoma from the pathology files of 14 institutions in 11 countries with the purpose of determining and correlating a simplified histological classification of thymoma and pathological staging with clinical outcome.
Molecular pathology selected abstracts
Ability of genetic alterations to predict development of acute myeloid leukemia
September 2018—Acute myeloid leukemia affects more than 60,000 people in the United States every year and has a mortality rate of more than 90 percent. It is the most common form of acute leukemia and is caused by unchecked growth of immature precursor cells in the bone marrow. These immature cells, or blasts, are myeloid precursors that often develop into dysfunctional, cancerous white blood cells that fill the bone marrow and spread into the blood.
Newsbytes
Nebraska informaticians mine and translate genomic data
September 2018—More than five years have elapsed since clinicians at the University of Nebraska Medical Center approached the institution’s informatics department with a problem. They wanted to more easily access structured genomic data stored in the EHR system for the diagnosis of cancer patients.
Letters
Burnout
September 2018—As a pre-med student, I am shadowing a pathologist mentor at a major cancer hospital to gain insight into the life of physicians. She introduced me to CAP TODAY. I write in response to “Frontline dispatches from the burnout battle,” by Karen Titus (June 2018), to share the thoughts of a novice who now knows that becoming a physician means learning the skill of resiliency as early as possible.
Microbiology’s shifting role in war on sepsis
August 2018—If you were casting about for the severest test of a laboratory’s capabilities, day in and day out, sepsis admissions at a pediatric hospital might fit the bill. At Children’s Hospital of Philadelphia, and at other hospitals, waging war on sepsis requires battles on multiple fronts and clinical pathways that rely on an agile and highly equipped microbiology laboratory. Three main categories of patients ensure there is no shortage of sepsis cases at CHOP, says Erin H. Graf, PhD, D(ABMM), director of the infectious disease diagnostics laboratory.
Read More »Addressing the gender gap: Women and burnout—like men, but not
August 2018—Jennifer Hunt, MD, gave it her best shot. While picking up her son after school one day (a task she only rarely had time for) and hearing staff ask, “Who are you?” yet again …
Read More »Serial NT-proBNP found to identify risk for adverse CV outcomes
August 2018—For diabetes type 2 patients with cardiovascular disease, findings of a new study support clinicians’ use of serial measures of NT-proBNP concentrations to make critical treatment decisions easier by basing them on risk of major cardiovascular events, including heart failure.
Read More »PGx testing: recommended alleles for CYP2C19 panels
August 2018—After more than a year of gathering information and deliberating, members of the Association for Molecular Pathology Pharmacogenomics Working Group have issued the first in what will be a series of recommendations to standardize pharmacogenetic testing.
Read More »Transfusion medicine checklist: Record and other requirements updated in new release
August 2018—One new requirement and several modified requirements in the CAP transfusion medicine checklist are part of the new edition of CAP accreditation program checklists released this month. In work led by the CAP Council on Accreditation, the checklists are examined anew and revised yearly, where needed. In transfusion medicine, the changes this year center on computer crossmatches, record retention, forward/reverse typing, and ABO group and Rh(D) type verification.
Read More »Cytology workload limits: For adequacy assessments, it’s time, not slides
August 2018—The CAP and the Centers for Medicare and Medicaid Services reached an understanding earlier this year on how adequacy assessments and rapid on-site evaluations in cytology can be accounted for without causing undue impact on workload limits. The agreement, communicated to state survey agency directors in a March 16 CMS memorandum, is reflected in the updated CAP accreditation program cytopathology checklist released this month.
Read More »Molecular lung cancer testing: from guideline to practice
August 2018—Testing turnaround times can affect whether non-small cell lung cancer patients receive an EGFR or ALK tyrosine kinase inhibitor when indicated. At disease progression on an EGFR TKI, integrating circulating tumor DNA and tissue-based testing may lessen some of the limitations of each form of testing.
Read More »Cytopathology in Focus: Synergy in cytopathology and molecular microbiology
August 2018—In today’s less-is-more world, health care consumers and providers often seek explicit and detailed information from minimally invasive procedures and tiny samples. Over are the days of “malignant cells present” and on to the next case. Cytopathologists and cytotechnologists are embracing and integrating novel techniques and applying new methods to the diagnosis and classification of essentially every imaginable form of neoplasia. The 2018 WHO publications confirm that 29 percent of deaths worldwide (more than 10 million people annually) are attributable to communicable diseases.1,2 This means the purpose of procuring many specimens is not to just rule out malignancy but also to diagnose infectious etiologies.
Read More »Cytopathology in Focus: Why not call everything ASCUS?
August 2018—Below is a question shared on the ASC listserv. My reply to the question follows. A pathologist colleague who practiced previously as an obstetrician/gynecologist is of the opinion that categorizing the level of abnormality we observe on a Pap test is a waste of time. All the clinician needs to know, he says, is whether the test is normal or abnormal. The Pap test is a screening test, he says correctly, and its only relevance is in pointing out who needs a colposcopy and biopsy.
Read More »Cytopathology in Focus: Cytology social media—Facebook and Twitter as networking tools
August 2018—If you are not already using social media professionally, you may not know there is a vibrant and active community of pathologists, including many cytopathologists, on Facebook and Twitter—and getting involved is easy, fun, and educational.
Read More »Put It on the Board
Cobas HPV test approved for first-line screening using SurePath preservative fluid
August 2018—Roche received FDA approval for the Cobas HPV test to be used as the first-line screening test for cervical cancer in women 25 and older using specimens collected in SurePath preservative fluid. The Roche test is now the only HPV test approved for use as a primary screening test with both SurePath and ThinPrep PreservCyt Solution. It is approved for all of the screening indications supported by guidelines—primary screening in women 25 and older, reflex testing of unclear Pap test results in women 21 and older, and cotesting with a Pap test in women 30 and older—with both of the primary collection media types. “With this additional approval for the Cobas HPV Test, laboratories and clinicians now have an approved option that can be used for all of their HPV screening indications and sample types,” Ann Costello, head of Roche tissue diagnostics, said in a statement.
Q&A column
Q. What is the best way to report fecal fat testing? Read answer. Q. Who developed the formula for the corrected white blood cell count for nucleated red blood cells, and how was the formula established? Read answer.
Read More »From the President’s Desk: More influence in the AMA House
August 2018—This year, I made a point of noticing just how much goes on during the AMA Annual Meeting so I could tell you about it. There are 600 delegates now and an equal number of alternates. More special sections give us more ways to engage, and we do. It’s challenging, enjoyable, and exhausting at once.
Read More »Clinical Pathology Abstracts
Association of perioperative RBC transfusions with venous thromboembolism
August 2018—Hospital-associated venous thromboembolism is a major cause of morbidity and mortality, resulting in 100,000 to 200,000 deaths annually. Surgery can lead to a proinflammatory state and be a prothrombotic stimulus for venous thromboembolism (VTE). General anesthesia, as well as red blood cells transfused in the perioperative setting, is considered an independent risk factor for VTE.
Anatomic Pathology Abstracts
Analysis of the surveillance of women diagnosed with atypical ductal hyperplasia on core needle biopsy
August 2018—A needle core biopsy diagnosis of atypical ductal hyperplasia is an indication for open biopsy. The launch of randomized clinical trials of active surveillance for low-risk ductal carcinoma in situ leads to the paradoxical situation of women with low-grade ductal carcinoma in situ being observed and those with atypical ductal hyperplasia having surgery.
Molecular Pathology Abstracts
Prevalence of clonal hematopoiesis mutations in tumor-only clinical genomic profiling of solid tumors
August 2018—Challenges to implementing next-generation sequencing-based comprehensive molecular profiling of solid tumors include reliably separating germline variants from somatic variants. This is an important consideration, particularly when a “tumor-only” profiling approach is used.
Newsbytes
New digital pathology certificate program educates ‘from A to Z’
August 2018—While there’s a lot of buzz about the growth of digital pathology, its steep learning curve is a potential impediment to implementation. Recognizing this, the National Society for Histotechnology, in collaboration with the Digital Pathology Association, launched in May a web-based digital pathology certificate program designed to provide both a broad overview of digital pathology and a deep dive into the details.
Small groups, big answers in HER2 testing
July 2018—Take the new ASCO/CAP guideline for HER2 testing. Since the first groundbreaking joint guideline appeared 11 years ago, the authors have made a habit of addressing cases that flummox pathologists, medical oncologists, and patients. Now, in 2018, they have clarified the diagnostic approach to in situ hybridization groups two, three, and four, rare cases that nonetheless cause an outsized share of headaches and worries. It also clarifies language from the 2013 guideline that had sent some labs astray, and it addresses the use of multiple alternative chromosome 17 probe assays. The previous guidelines turned out to be tough acts to follow—a bit like following Sean Connery in the role of James Bond—even as the new one benefits from new data.
Read More »Look, wait, buy: labs share instrument plans
July 2018—“Robbie,” the autonomous service robot that transfers specimens for Florida Hospital’s central laboratory, may not quite be ready for his gold watch. But after five years of faithful service delivering samples between the different esoteric testing units, he’s nearing the end of his natural lifespan with signs of wear.
Read More »With CMS coverage policy, NGS cancer testing goes large
July 2018—The March 16 announcement of a new Centers for Medicare and Medicaid Services coverage policy for next-generation-sequencing–based diagnostic lab tests for patients with advanced cancer did not appear out of the blue, since a draft policy was issued last fall.
Read More »Artificial intelligence: what’s possible, why now?
July 2018—When it comes to artificial intelligence, it can be difficult to distinguish hyperbole from reality. So how much can AI truly replace human tasks in society and, more specifically, in medicine?
Read More »In memoriam: Harold H. Harrison, MD, PhD (1951–2018)
July 2018—Harold H. Harrison, MD, PhD, 67, Pennsylvania state commissioner in the CAP Laboratory Accreditation Program and a member of the Inspection Process Committee, died suddenly June 6 of cardiac causes. Dr. Harrison joined the Geisinger Health System in Danville, Pa., in 2007 where he was director of clinical pathology and director of Geisinger Regional Laboratories.
Read More »For pain care and more, PGx testing at Avera Health
July 2018—Putting pharmacogenetic testing into play at Avera Health was years in the making. It took time to operationalize it at an affordable cost. Today, it has wide physician acceptance and is seen as a strong benefit for patients. “Pharmacogenetics is what will differentiate Avera in a new era of ACOs and personalized medicine, and will ultimately lead to a model for transforming health care,” says Trisha Lauterbach, MS, MLS(ASCP)CM, laboratory operations manager at Avera Institute for Human Genetics (AIHG), Sioux Falls, SD.
Read More »Direct oral anticoagulants and APTT, PT results: The risk of normal results in patients on therapy
July 2018—With the introduction of direct oral anticoagulants (DOAC) there is a paradigm shift in the use and understanding of screening coagulation tests to determine a patient’s bleeding risk. In patients on DOAC therapy, clinicians cannot rely on normal activated partial thromboplastin time (APTT) and prothrombin time (PT) results to reflect the patient’s level of anticoagulation.
Read More »Menu, security, consistency: vendors point to priorities
July 2018—Chemistry and immunoassay analyzers combined—that’s what is new about the product guide. In years past, the chemistry and immunoassay analyzer product guides were published separately. This year we integrated them and are publishing them in two issues.
Read More »Put It on the Board
High-sensitivity troponin I assay available in the U.S.
July 2018—Beckman Coulter Diagnostics received 510(k) clearance from the Food and Drug Administration for its new high-sensitivity troponin assay, Access hsTnI, for use on the Access 2, DxI, and the entire Access family of immunoassay systems. Access hsTnI demonstrates less than 10 percent CV at the upper reference limits for men and women and detects troponin in more than 50 percent of the healthy population. In an independent study, Access hsTnI detected more than 99 percent of troponin values for healthy men and women (Pretorius CJ, et al. Clin Biochem. 2018;55:49–55). “Beckman Coulter’s high-sensitivity cardiac troponin I assay can measure very low cardiac troponin concentrations with excellent precision. This test may help physicians with both the early diagnosis of myocardial infarction and future risk stratification in and outside the acute coronary syndrome setting,” Peter Kavsak, PhD, associate professor, Department of Pathology and Molecular Medicine, McMaster University, said in a statement.
Clinical Pathology Abstracts
Trends in perioperative RBC transfusion from index cases in five surgical specialties
July 2018—In recent years, greater attention has been given to patient blood management. While contemporary national guidelines recommend restrictive red blood cell transfusion, it is not known whether such transfusions have decreased in surgical patients. Approximately 11 million RBC transfusions are performed annually, and two-thirds of those are for patients in the perioperative period.
Q&A column
July 2018—Is CD30 currently being used as a predictive marker for therapy? Due to laboratory construction, our molecular instruments were relocated within the lab. Is full test validation required in this case? Or is running at least 20 known samples enough to verify the instrument/assay performance specifications?
Read More »President’s Desk: From concept to fruition
July 2018—CAP members know that laboratory quality improvement and accreditation drive much of what we do. Nobody can know everything about the science underlying our specialty because pathology embraces a vast body of knowledge that is always changing …
Read More »Newsbytes
July 2018—How a geospacial tracking system is closing the distance at Michigan lab: This summer, when the Michigan Medicine Department of Pathology moves the last of its laboratories to a new campus four-and-a-half miles from the existing University Hospital laboratories in Ann Arbor, it will kick its new PathTrack Lean-influenced real-time geospatial tracking system into high gear. Developing the system, which will monitor an anticipated 6,000 to 10,000 specimens every day, has been “a monumental effort,” says Ulysses J. Balis, MD, director of the health system’s division of pathology informatics.
Read More »Anatomic pathology Abstracts
Clinical and molecular analyses of neuroendocrine carcinomas of breast
July 2018—Clinical and molecular analyses of neuroendocrine carcinomas of breast: Neuroendocrine breast carcinomas represent a rare subtype of breast cancer. Their definition, prevalence, and prognosis remain controversial, as reported in the literature.
Molecular pathology selected abstracts
July 2018—Correlation between tumor mutation burden and efficacy of combination immunotherapy in nonsmall cell lung cancer: Checkpoint inhibitor therapy has dramatically improved outcomes in many cancer types, with treatments including antibodies against cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), programmed cell death receptor-1 (PD-1), and its ligand (PD-L1).
Read More »Letters
July 2018—I was excited to see the article “Clearing the air for electronic cancer checklists” (May 2018) and would like to share what we at Sunquest are doing to contribute to adoption of the electronic cancer checklists. Sunquest is continually improving the PowerPath Synoptics tool set, which is included as part of our base system.
Read More »Frontline dispatches from the burnout battle
June 2018—Bryan Bohman, MD, doesn’t spend his days wandering the Bay Area handing out buttons that read “Lift people, not the bottom line.” But don’t rule this out as a possibility someday, either. Dr. Bohman, chief medical officer, University Healthcare Alliance, and clinical professor of anesthesiology and perioperative and pain medicine, Stanford Health Care, is campaigning against physician burnout. Yes, it threatens the quality of medical care, he says, and yes, it’s expensive.
Read More »For one laboratory, a workflow transformation
June 2018—“Form follows function” is a famous design principle, coined by an architect. But in the health care system and elsewhere, perfect matches between form and function are scarce.
Read More »NGS to take top spot as cancer biomarker testing broadens
June 2018—For biomarker testing and tissue conservation, all roads lead to next-generation sequencing, says Boaz Kurtis, MD, laboratory and medical director of Cancer Genetics in Los Angeles. Dr. Kurtis said, “There’s no other technology platform out there that can provide the amount of data we need today or will need in the future.”
Read More »Questions about frozen tissue, preanalytic variables, tumor content
June 2018—Boaz Kurtis, MD, laboratory and medical director of Cancer Genetics in Los Angeles, took the following questions from attendees at a webinar on NGS in routine non-small cell lung cancer biomarker testing.
Read More »Satisfaction high with new automated AMH assays
June 2018—Testing for anti-müllerian hormone got a boost in 2017 when the year began and ended with the FDA clearing the first two fully automated AMH assays from Roche and Beckman Coulter.
Read More »Core needle biopsy of the breast: cases and cautions
June 2018—With core needle biopsies of the breast, if something looks like an epithelial malignancy, ask yourself: Is it really a carcinoma? If it is a carcinoma, ask yourself if it is a primary breast carcinoma.
Read More »Instrument acquisition, skilled labor on the table: Makers of chemistry, immunoassay analyzers answer our questions
June 2018—Broad menus, efficient workflows, single platforms, rural labs, cybersecurity, and economics are some of what one CEO and three marketing and product managers talked to CAP TODAY about when we spoke to four companies whose analyzers are profiled in the product guide.
Read More »Q&A column
June 2018—What is the role of total testosterone and free testosterone in gauging the effectiveness of androgen deprivation therapy?
We are planning to validate the mismatch repair panel in our immunohistochemistry laboratory. Do we use the CAP guidelines for antibody validation for a nonpredictive marker or a predictive marker?
New reference guide for ultrasound-guided FNA
June 2018—CAP Press released last month a new reference guide, Ultrasound Features of Superficial and Palpable Lesions. It’s small and spiral bound and has a laminated cover and tabs for easy reference. There are 375 images and illustrations in the guide’s 200 pages.
Read More »Clinical Pathology Abstracts
June 2018—Multiple myeloma and precursor disease in firefighters at World Trade Center disaster; Association between time to colonoscopy after a positive fecal test and colorectal cancer
Read More »Anatomic Pathology Abstracts
June 2018—Impact of pattern of invasion in invasive endocervical adenocarcinoma; Inflammatory myofibroblastic tumor of the uterus: an analysis of 13 cases.
Read More »Molecular Pathology Abstracts
June 2018—Genetics and pathogenesis of diffuse large B-cell lymphoma: Understanding the genetic basis of diffuse large B-cell lymphoma is important for understanding the pathogenesis of the disease and the molecular attributes that may influence therapeutic response.
Read More »Newsbytes
June 2018—Electronic device shows promise for identifying pathology specimens: While barcodes and radio-frequency identification are considered the workhorses of pathology specimen identification, a new technology, nearly two decades in the making, may soon get a piece of the action.
Read More »Put It on the Board
June 2018—FDA clears T2Bacteria panel for detecting sepsis-causing pathogens: T2 Biosystems received market clearance from the Food and Drug Administration for the T2Bacteria panel for the direct detection of bacterial species in human whole blood specimens from patients with suspected bloodstream infections.
Read More »Letters
June 2018—LCIS variants and DCIS: We write in response to the article by Karen Lusky regarding tips to distinguish DCIS from variant forms of LCIS (April 2018). A different question might be: Is it actually important to distinguish these two in situ proliferations?
Read More »From the President’s Desk—CAP18: bringing it all back home
June 2018—Nobody has an annual meeting like ours. Nobody. And CAP18 will be no exception. The CAP18 educational program features 85 courses presented by 128 outstanding faculty. A quick scan of the program reveals 42 new learning opportunities and another 38 returning by popular demand.
Read More »Skirting the pitfalls of merging lab results
May 2018—“One of these things is not like the others” is a fun puzzle for kids in the context of Sesame Street. But it can be a vexing informatics challenge when you are managing data entered in fields in a database. For anyone charged with merging outside laboratory results into an institution’s electronic health record alongside results from an in-house laboratory, the differences can generate no end of headaches.
Read More »New contender speeds ID with susceptibility testing
May 2018—If a seismic shift were to happen in microbiology, the technology behind the Accelerate Pheno system and PhenoTest BC test kit, which won FDA approval last year as a rapid pathogen identification and antimicrobial susceptibility testing (AST) system for blood cultures, could well be the cause.
Read More »Teaming up: how one site is managing its complex liver cases
May 2018—It didn’t take long for Heather Stevenson-Lerner, MD, PhD, to grasp one key fact about the liver biopsy cases she was seeing at the University of Texas Medical Branch, Galveston: They were often complicated. UTMB sees plenty of challenging liver cases of its own, says Dr. Stevenson-Lerner, assistant professor of medicine and liver and transplantation pathologist, Department of Pathology.
Read More »Pharmacogenomics advocates make case for wider use
May 2018—Use of pharmacogenomic testing is still limited, despite ample research, the existence of guidelines, and the emerging evidence it can help patients. Panels can be costly and insurance coverage variable, and providers need guidance—from pharmacists, the lab, decision support alerts—in knowing what and when to order and in understanding the results. Plus, patients move.
Read More »From the President's Desk: What we learn from member surveys
May 2018—As a professional society, we want to know what our members need so we can provide services and programs to help them excel. We know that pathology practices are diverse because they have to be—science is never static. We also know that practice settings vary widely. In short, CAP members’ interests and concerns are uncommonly diverse because our field is uncommonly diverse.
Read More »Clearing the air for electronic cancer checklists
May 2018—Length, cost, variability in vendor support, and lack of consistency have cast a cloud for pathologist users over the CAP’s cancer protocols and the electronic version of those protocols, the electronic cancer checklists. Work is underway to improve the user experience (Nakhleh RE, et al. Arch Pathol Lab Med. 2017;141[9]:1153–1154). Behind that effort is the undeniable: “Structured discrete data, using a controlled vocabulary, can be captured, stored, and reviewed much more readily than data in other formats,” says Mary Edgerton, MD, PhD, vice chair of the CAP’s Pathology Electronic Reporting (PERT) Committee and associate professor of pathology, University of Texas MD Anderson Cancer Center.
Read More »With hemolysis, tackling the rush with the reasoning
May 2018—First a journey. Then sometimes a vigorous shake. Little wonder that red blood cells hemolyze. “Red blood cells don’t like to be stressed,” says Kathleen Finnegan, MT(ASCP)SH, phlebotomy training program director at Stony Brook University School of Technology and Management, New York. She instructs her students to avoid stressing the RBCs by skipping what she calls the “martini shake” (CLSI recommends five to 10 tube inversions), using a needle that is the right size, and not using a syringe for transfer but instead a transfer device. “So it’s gentle,” she says.
Read More »Cytopathology in Focus: Reporting salivary gland cytopathology—new user-friendly Milan system consists of six diagnostic categories
May 2018—The Milan System for Reporting Salivary Gland Cytopathology was published Jan. 31 and is an important step toward standardizing the reporting of salivary gland fine needle aspiration. A large body of literature has demonstrated that FNA is an effective method for the initial evaluation of salivary gland masses, but until this year there was no uniform, widely accepted reporting system. The complexity of salivary gland cytology poses unique challenges that demand a standardized approach to communication of diagnostic information between pathologists and treating clinicians.
Read More »Cytopathology in Focus: For thyroid cytopathology, the 2017 Bethesda System
May 2018—Surgical pathologists take their tumor nomenclature from the WHO Classification of Tumours, but cytopathologists take their terminology from where the consensus groups convened—Bethesda, Paris, Milan, and Yokohama—to formulate terminology recommendations. The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC)1 is now in its second edition.
Read More »Clinical Pathology Abstracts, 5/18
May 2018—Use of a smartphone app to assess neonatal jaundice: Neonates are screened for hyperbilirubinemia before hospital discharge using a transcutaneous or total serum bilirubin measurement. However, levels peak at approximately 96 hours of life, which is after most healthy infants have left the hospital. Outpatient follow-up is often performed by visual inspection, but this can be highly variable and have poor interobserver agreement.
Read More »Anatomic Pathology Abstracts, 5/18
May 2018—Outcomes related to use of hygroscopic sonographically detectable clips: The use of hygroscopic sonographically detectable clips (HSDCs) has dramatically increased in recent years, especially for breast cancer patients who undergo neoadjuvant chemotherapy. The authors conducted a study to define the appearance of HSDC sites in histopathological specimens and allow pathologists to recognize these sites and differentiate them from other lesions.
Read More »Molecular Pathology Abstracts, 5/18
May 2018—DNA methylation-based testing to classify central nervous system tumors: Despite being the mainstay of pathology tissue diagnostics, microscope-based histological review has limitations. Among them is that pathologists may have differing opinions about a case. This interobserver variability may result in over- or undertreatment of the patient and lack of agreement about which diagnosis is correct.
Read More »Newsbytes, 5/18
May 2018—Vendor neutral archives: A fit for the pathology lab? Whether the initialism VNA will become as recognizable as the acronym PACS in the pathology field remains to be seen, but pathologists and vendors alike are considering how vendor neutral archives may benefit the pathology lab.
Read More »Q&A column, 5/18
May 2018—Our immunohistochemistry laboratory is moving to a new building across the street. We are not getting new equipment, just moving the machines to the new building. Do we need to perform a full revalidation of all our antibodies?
Read More »Put It on the Board, 5/18
May 2018—FDA approves osimertinib for NSCLC, nivolumab + ipilimumab for RCC: The FDA on April 18 approved osimertinib (Tagrisso, AstraZeneca) for the first-line treatment of patients with metastatic non-small cell lung cancer whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations, as detected by an FDA-approved test.
Read More »Q&A column
April 2018—A semen analysis for viability was collected at 9:30 AM and not received in the laboratory until 1:40 PM. Our standard operating procedure says this test must be analyzed one hour after collection, with no disclaimers stated for late receivables. Therefore, it is my understanding that a specimen received five hours after collection would be considered unacceptable because the viability of the semen is compromised and the collection delivery does not follow our SOP.
Read More »TB testing: new approaches to old scourge
April 2018—Scratch the surface of TB testing, and things quickly get interesting. The standard skin reaction test, widely adopted by the early 1940s, is still in use today. The goal has remained steady as well: break the transmission cycle. “From the clinician perspective and the laboratory perspective, because of its infectious nature, we want to identify people with latent tuberculosis,” says Elitza Theel, PhD, lab director for the infectious disease serology laboratory, Mayo Clinic and Mayo Medical Laboratories.
Read More »HBsAg tests, mutation in public health spotlight
April 2018—If you were asked to pick a place on the map where problems with detecting a mutant strain of an infectious disease would likely come to light, the capital of Nebraska might not be your first guess.
Read More »Digital pathology: A 1st anniversary report card
April 2018—Nearly one year after the FDA cleared the Philips IntelliSite Pathology Solution for primary diagnosis, Philips is reporting worldwide momentum for the adoption of digital pathology.
Read More »Fewer false-positive pregnancy results with intact hCG
April 2018—When women of childbearing age check in at a cancer center where they might be undergoing medical or surgical treatment, the screening protocol is often to test them for pregnancy, primarily by quantifying serum β-hCG.
Read More »From the President’s Desk: Pathologists as medicine’s first responders
April 2018—The National Institute on Drug Abuse estimates that 100 million Americans suffer from chronic pain. The majority of drug overdose deaths involve an opioid, and nearly half of drug overdoses caused by opioids involve prescription drugs.
Read More »LCIS variants and DCIS: tips on telling them apart
April 2018—DCIS or LCIS? Making the distinction can be difficult in some cases. Stuart J. Schnitt, MD, in a session at CAP17 on ancillary testing in breast pathology, delineated the reasons and provided tips, including the role of E-cadherin immunostains to help in this distinction. The cells of DCIS typically show strong membrane staining for E-cadherin while the cells of LCIS are typically E-cadherin negative. But among the tips: If an in situ lesion is E-cadherin positive, it doesn’t automatically mean it’s ductal carcinoma in situ. As he demonstrated in several cases, the lesion could be lobular carcinoma in situ with aberrant E-cadherin immunostaining.
Read More »Turnover in phlebotomy: looking deeper than pay
April 2018—Laboratory managers struggling to reduce turnover among phlebotomists should look beyond the pay and examine their hiring and management practices and the dysfunction that could be creating walls between analytical and preanalytical staff. “It’s an enormous problem,” Dennis J. Ernst, MT(ASCP), NCPT(NCCT), director of the Center for Phlebotomy Education, says of phlebotomist turnover. “There’s no silver bullet because there are so many things that lead phlebotomists to give up hope where they work and in the profession. It’s critical that managers are tuned in to the needs of this specialized workforce because they’re varied and many.”
Read More »POC glucose: views on volume, critical care, ACOs
April 2018—Test volume, limitations on devices used in critical care, consolidation, and population health is what CAP TODAY asked about when it spoke in March with the makers of three bedside glucose testing systems. Their systems and those of two other companies are profiled on pages 44-49. “The customers are more aware than ever of the limitations that are in the package inserts from the glucose manufacturers,” says Corrine Fantz, PhD.
Read More »In cervical disease dx, agreement rises with p16 IHC use
April 2018—In analyzing cervical tissue, adjunctive use of p16 IHC with H&E-stained slides improves accuracy and sensitivity, according to the results of the Cervical Tissue Adjunctive Analysis study presented by Thomas C. Wright Jr., MD, in a webinar hosted by CAP TODAY and made possible by an educational grant from Roche Diagnostics.
Read More »Clinical Pathology Abstracts
April 2018—Potential contributors to error in oxygen saturation calculation using a POC assay: Oxygen saturation is important for measuring respiratory status and calculating cardiac output for patients.
Read More »Anatomic Pathology Abstracts
April 2018—Confirmation of ProMisE: a clinical classifier for endometrial cancer; Leiomyoma with bizarre nuclei: a morphological, IHC, and molecular analysis.
Read More »Molecular Pathology Abstracts
April 2018—Effect of inherited TP53 mutations on children with B-cell ALL: TP53 has been referred to as the “guardian of the genome” because it plays a central role in regulation of the cell cycle, DNA repair, and apoptosis, and because somatic mutations in TP53 are frequently identified in many tumor types.
Read More »Newsbytes, 4/18
April 2018—Data-extraction system demonstrates potential for pathology laboratories: Just as parents instill in their children a desire to improve themselves, in part through interactions with others, some software developers are “teaching” their tools to interact and adjust accordingly.
Read More »Put It on the Board, 4/18
April 2018—CMS changes proposed policy on NGS for cancer patients: The CAP and the Association for Molecular Pathology, in separate statements in March, lauded the Centers for Medicare and Medicaid Services for revising its national coverage determination on next-generation sequencing.
Read More »Letters, 4/18
April 2018—Let us bring back breast FNAB: The article “Standardized reporting for breast FNAB cytology” (January 2018) is a welcome account of some of the challenges associated with breast fine needle aspiration biopsy compared with core needle biopsy. The author expertly outlines the significance of a standardized approach to performance, interpretation, and reporting of the breast FNAB procedure. The article was a pleasant surprise because the topic of breast FNAB has not been the subject of much interest in our pathology community during the past several years.
Read More »Lung guideline goals: more tests, treatment
March 2018—Among the many never-ending chores that humans undertake—paying bills, filing taxes, flossing—writing medical guidelines can seem like an especially perpetual task. Just ask the architects of an updated document on molecular testing for lung cancer, issued by the CAP, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology.
Read More »Labs take stock of surprising flu season
March 2018—In a severe flu season that started early, laboratories faced unprecedented test volumes, used new testing platforms, and negotiated vendor supply shortages. When laboratory staff at Arkansas Children’s Hospital in Little Rock began seeing a rising number of requests for respiratory tests, and five positive flu results,
Read More »Puzzling out the positive shift in the final 14-day rule
March 2018—When the CMS’ new 14-day rule took effect Jan. 1, conditions for laboratories doing outpatient reference testing might have changed for the better. But for labs navigating the new billing regulations, some forecasters are predicting confused seas ahead. “We’ve been reaching out to a number of our customers who I know will be affected by this and saying ‘What’s your take?’ and together just putting our heads around what it really means. But there is still quite a bit of confusion out there,” says Kurt Matthes, vice president, reengineering and service, at revenue cycle management software provider Telcor.
Read More »Inflammatory biomarkers foreshadow CKD, study finds
March 2018—The central idea of the film Minority Report—that a “precrime” police unit can predict and prevent crimes—still mostly inhabits the realm of science fiction. Luckily, in medicine, researchers studying “predisease” can make headway on prevention by analyzing the laboratory test results from samples collected years earlier, when patients showed no clinical symptoms, that might have been able to predict disorders such as chronic kidney disease (CKD) in those patients.
Read More »From the President’s Desk: A warm welcome around the world
March 2018—I was just out of training and still getting my bearings when I learned that the new partner in our group was expected to manage CAP Laboratory Accreditation Program inspections. Today I think that is a good idea, but back then I was nervous. I had done several CAP inspections as a resident. But did I know enough to get a laboratory through one?
Read More »Why carbapenemase-producing CRE raise the bar
March 2018—When Stephen Brecher, PhD, compares MRSA to carbapenem-resistant Enterobacteriaceae, the figures of speech come fast and furious. “We are not in Kansas anymore,” he said. “The bar has been raised. I consider MRSA a picnic compared to CP-CRE [carbapenemase-producing CRE].”
Read More »Pros and cons of carbapenemase detection tests
March 2018—When it comes to diagnostic tests, everyone wants the same thing Lars Westblade, PhD, wants: A unicorn. “The diagnostic performance of a test is reflected in its sensitivity and specificity,” Dr. Westblade said. “It has to be a very good test. And then we need to think about the speed of the test.” There’s also the cost. When all these factors come together just so, “we get what’s called diagnostic perfection,” he says, or the rare event that Brandi Limbago, PhD, of the CDC calls “a diagnostic unicorn.”
Read More »For AP signout: infectious diseases pathology atlas
March 2018—New from CAP Press this month is the Atlas of Fundamental Infectious Diseases Histopathology: A Guide for Daily Practice, edited by Bobbi S. Pritt, MD, MSc, DTM&H. It covers bacterial, viral, fungal, and parasitic infections and contains more than 800 images.
Read More »Gene testing moves cardiomyopathy analysis forward
March 2018—From phenotype to genotype in the understanding and diagnosis of cardiovascular disease—that was the medical journey on which Joseph Maleszewski, MD, and Birgit Funke, PhD, took attendees at a symposium at the November 2017 meeting of the Association for Molecular Pathology.
Read More »Clinical Pathology Abstracts, 3/18
March 2018—Web platform vs. genetic counselor for releasing carrier results from exome sequencing: Genomics can be used to generate a large amount of data that may have important implications for clinical care and selection of therapeutics. However, a bottleneck exists in clinical genomics due to the large volume of results and the lack of availability of knowledgeable professionals to return them to patients in person.
Read More »Anatomic Pathology Abstracts, 3/18
March 2018—Magee equation 3 for predicting response to chemotherapy in some breast tumors: Magee equations were derived as an inexpensive, rapid alternative to the Oncotype DX commercial assay. Magee equation 3 uses immunohistochemical and FISH data for estrogen receptor (ER), progesterone receptor (PR), HER2, and Ki-67 for its calculation: 24.30812+ERIHC×(–.02177)+PRIHC×(−0.02884)+(0 for HER2 negative, 1.46495 for equivocal, 12.75525 for HER2 positive)+Ki-67×0.18649.
Read More »Molecular Pathology Abstracts, 3/18
March 2018—Nonendoscopic detection of Barrett’s esophagus using DNA methylation biomarkers: Esophageal adenocarcinoma is an aggressive disease, with a less than 20 percent five-year survival rate, and its incidence is rapidly increasing. Early detection of esophageal adenocarcinoma or its precursor lesion, Barrett’s esophagus, would enable more effective treatment strategies and a greater chance of cure.
Read More »Newsbytes, 3/18
March 2018—How hospitals use savvy and software as a phishing net: We all know we shouldn’t click on suspicious emails, but suppose you see an email from your department of human resources with an attached document about a new dress code. You open it, thinking “What new dress code?” And now you’ve infected the hospital’s computer system with a virus.
Read More »Q&A column, 3/18
March 2018—Our pathology group has an unusual case of residual squamous cell carcinoma of the lung in a lobectomy specimen after chemotherapy. The lung shows a hilar scar (1.7 cm) involving the lung parenchyma and the peribronchial adipose tissue. In the scar there is residual carcinoma (0.4 cm) that focally is involving the peribronchiolar adipose tissue around the lobar bronchus. The focus is located at 0.3 cm of the final surgical resection margin of the bronchus. Because the tumor involves peribronchiolar adipose tissue, is it considered outside the lung (extension outside the lung)? Since the tumor is in the mediastinal fat around the bronchi and had to invade the viscera pleura to invade the peribronchial adipose tissue, would the tumor stage be ypT2a? Or T3 since it is invading part of the mediastinal fat? Or should it be pT1?
Read More »Put It on the Board, 3/18
March 2018—AMP issues recommendations for clinical CYP2C19 genotyping allele selection: To promote standardized testing across laboratories, the Association for Molecular Pathology published on Feb. 27 consensus, evidence-based recommendations for designing and validating clinical CYP2C19 assays.
Read More »New HPV guideline for head, neck cancers
February 2018—Like a pair of one-size-fits-all jeans, testing all head and neck carcinomas for human papillomavirus may have seemed like a good idea at one time. In many cases, in fact, HPV testing is just what treating clinicians and patients need. On the other hand, not every head and neck case requires it. Now, a newly published CAP guideline should help physicians figure out the right fit in multiple settings.
Read More »Scoring gastric, GEJ cancers for PD-L1 expression
February 2018—To some ears, perhaps, the scientific method connotes a process that is standardized and unimaginative. But inventions like Velcro, vulcanization, and the microwave—all stemming from accidental discoveries—testify to the role of luck and leaps of intuition in formulating and modifying a hypothesis.
Read More »Molecular tumor board: a patient with ALK– rearranged lung cancer
February 2018—A case of ALK-rearranged lung cancer was the subject of a multidisciplinary molecular tumor board presented last fall at CAP17 by pathologist Laura J. Tafe, MD, and oncologist Benjamin Levy, MD. Together they offered up insights into the tumor genomics of lung cancer with talk of testing guidelines, targeted therapies, resistance mechanisms, and circulating tumor DNA analysis.
Read More »Smart test ordering—new program provides the tools
February 2018—A new CAP program with a novel approach makes it easier to take on an old problem: misapplied laboratory tests. The CAP Test Ordering Program, available now and complimentary to all members, is different from other laboratory test utilization initiatives, says Richard W. Brown, MD, medical director for system laboratory services at Memorial Hermann Health System in Houston.
Read More »From the President’s Desk: Human factors engineering
Last month we talked about the eighth edition of the American Joint Committee on Cancer staging manual, which was launched Jan. 1. CAP experts had undertaken a full-court press to harmonize our cancer protocols with the new edition.
Read More »AMP case report: Detection of rare deletion mutation in the alpha-globin gene locus establishes a diagnosis of Hb H disease
February 2018—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Quest Diagnostics. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Read More »Procedures up to date? Fighting injury in phlebotomy
February 2018—Requiring strict adherence to the latest industry standard for venipuncture can go a long way to minimizing the risk of phlebotomy-related lawsuits and multimillion-dollar jury awards. “It revolves right back to education,” says Nancy Erickson, PBT(ASCP), an expert witness in more than 30 phlebotomy-related lawsuits. She says lack of education and failure to follow the standard of care cause the two most common patient complaints that lead to phlebotomy-related litigation: nerve damage and syncope.
Read More »AP-LIS vendors talk reports, interfaces, protocols
February 2018—Customer demand, cancer protocols, and consolidation of pathology practices are some of what CAP TODAY asked about when it spoke in January with four anatomic pathology computer system companies. Their AP systems and those of 17 other companies are profiled in the anatomic pathology computer systems interactive product guide. “It’s a really good time for our market right now,” says Joe Nollar of Xifin, “and systems providers need to be creative in helping their clients get the solutions they need to be scalable, competitive, and profitable.” Here is more of what they told writer Anne Ford.
Read More »For precision medicine, next-generation mass spec
February 2018—The modern analytical technologies of mass spectrometry continue to garner prominence and broader utility in clinical diagnostics. This was showcased at the 7th Annual American Association for Clinical Chemistry Conference on Mass Spectrometry and Separation Sciences for Laboratory Medicine, held last fall in Philadelphia. Representatives of academia, industry, and regulatory bodies came together to share information about the technology and best practices, the aim of which is to strengthen clinical diagnostics for the betterment of patient care. In opening remarks, then CAP president Richard Friedberg, MD, PhD, shared his hopes for the future of mass spectrometry in anatomic pathology.
Read More »Clinical Pathology Abstracts, 2/18
February 2018—Restrictive or liberal approach to red blood cell transfusion for cardiac surgery: Among the largest group of recipients of red blood cell transfusions are patients undergoing cardiac surgery. Whether a restrictive approach to intraoperative and postoperative transfusion in cardiac surgery is superior to a more liberal approach with regard to patient outcomes is unclear.
Read More »Anatomic Pathology Abstracts, 2/18
February 2018—ARTEMIS trial: quantifying pathological response to neoadjuvant chemotherapy: The Affordability and Real-World Antiplatelet Treatment Effectiveness after Myocardial Infarction Study, also known as the ARTEMIS trial, tested standard neoadjuvant chemotherapy with or without bevacizumab in the treatment of HER2-negative early breast cancer.
Read More »Molecular Pathology Abstracts, 2/18
February 2018—Gene expression and risk of leukemic transformation in myelodysplasia: The myelodysplastic syndromes represent a group of clonal hematopoietic disorders with varying prognoses, with survival ranging from a few months to more than 10 years. Multiple laboratory measurements have been used in attempts to provide reliable prognostic assessments, including bone marrow blast counts, severity of peripheral cytopenia, cytogenetic findings, and, most recently, gene-mutation profiling.
Read More »Newsbytes, 2/18
February 2018—The many facets of a laboratory IT budget: Creating an information technology budget for the laboratory may seem like a fairly straightforward, if painstaking, exercise. But a number of factors that can affect the laboratory’s bottom line are frequently overlooked during the budgeting process, according to two health care consultants who spoke with cap today.
Read More »Q&A column, 2/18
February 2018—I come from a core (hematology/chemistry) background, and I would like practical, how-to guidance in developing an effective QC strategy for HIV viral load testing. What performance characteristics do you verify? How many and what type of samples do you use? What are the chosen acceptable thresholds? Do you use L-J charts? If so, what do you plot, what control rules do you select, and how do you select them?
Read More »Put It on the Board, 2/18
February 2018—PreludeDx unveils predictive assay for DCIS: PreludeDx announced results from its oral presentation at the San Antonio Breast Cancer Symposium. The results using the SweDCIS randomized trial confirmed that the DCISionRT test predicts which patients with ductal carcinoma in situ will benefit from radiation therapy after breast-conserving surgery.
Read More »Clin Lab 2.0: Add value, make patients better
January 2018—It was baseball’s Yogi Berra who said, with the unique slant that was his hallmark, “In theory there is no difference between theory and practice. In practice, there is.” More vividly, boxer Mike Tyson once summed up the same reality when asked to comment on an opponent’s strategy in an upcoming match: “Everybody has a plan—until they get hit.”
Read More »Genotype-guided dosing of warfarin: GIFT wrap-up
January 2018—In an ideal world, clinical research data would be applied with immediate and beneficial effect to clinical practice, especially when the data come from a well-controlled, well-run trial that meets the gold standard of being large, randomized, and blinded.
Read More »Next-gen sequencing finds further clinical utility in oncology
January 2018—One of the plenary sessions at the 2017 meeting of the Association for Molecular Pathology—“High Impact Molecular Diagnostics for Cancer and Inherited Diseases”—was a virtual mini-course in the latest and most useful applications of next-generation sequencing to detect germline and somatic mutations in cancer. Both speakers zeroed in on the clinical utility of their innovative diagnostic techniques.
Read More »New Color Atlas of Hematology to be used ‘in the wild’
January 2018—New this month from CAP Press is the second edition of the Color Atlas of Hematology: An Illustrated Field Guide Based on Proficiency Testing. “More and better are the watchwords,” senior editor Eric F. Glassy, MD, told CAP TODAY when we asked what the reader can expect. David Blomberg, MD, and Katherine Galagan, MD, are associate editors.
Read More »From the President’s Desk: A shared vision for cancer staging
January 2018—For me, friendships formed and lessons learned have more than compensated for the effort invested over the years on CAP committees, but make no mistake: When we meet, what we’re doing is work. The professional engagement is enjoyable, but a person can get tired toward the end of a two-day meeting, not to mention homework in the evenings.
Read More »Devices, decisions: POC glucose in the critically ill
January 2018—Using point-of-care glucose meters in critically ill patients can feel like tiptoeing through a regulatory minefield. Perhaps your preferred meter hasn’t been cleared by the FDA for use in this population. Or maybe you’re not sure which assay performance requirements should be regulating the performance of your meters. Or perhaps you’re still trying to define “critically ill.”
Read More »Lab needs driving coagulation analyzer market
January 2018—Customer wish lists help to define every generation of coagulation analyzers, test menus, and related technologies. That’s evident in the recent and upcoming launches and the ongoing work of the companies whose analyzers are profiled in this issue in the 2018 coagulation analyzer product guide.
Read More »AABB seeks comments on form to streamline transfusion adverse reaction reporting
January 2018—The AABB is seeking comments by March 30 on its common transfusion reaction reporting form, the seven pages of which are presented online at www.bit.ly/AABB-reportform. The fillable PDF form is intended to be used by hospitals and blood centers to communicate information about transfusion reactions to the blood supplier, particularly when there are multiple suppliers to the hospital transfusion service.
Read More »Cytopathology in Focus: Standardized reporting for breast FNAB cytology
January 2018—In countries with developed medical infrastructure, the use of breast fine-needle aspiration biopsy (FNAB) cytology has had its share of challenges over the past 20 years, among them the use of core needle biopsies. In developing countries where the use of FNAB cytology has been increasing rapidly, breast lesions are one of the most common sites sampled by FNAB. In 2016, the International Academy of Cytology Executive Council put together a “Breast Group,” which consists of cytopathologists, surgical pathologists, radiologists, surgeons, and oncologists working in breast care, with the aim of producing a comprehensive and standardized approach to breast FNAB cytology reporting.
Read More »Cytopathology in Focus: A right and a wrong way to use CAP educational kits
January 2018—The CAP Cytopathology Committee constructs educational and interlaboratory comparison kits that are distributed regularly to cytotechnologists, cytopathologists, and pathologists who want continuing education in cytopathology. The purpose of the kits is to make it possible for those who screen and diagnose cytology slides to maintain and update their skills. However, the Cytopathology Committee has been made aware that the kits have been employed for purposes other than education. We address here the potentially detrimental uses to which some laboratories are putting these educational kits and advise laboratories to use them only as they were intended.
Read More »Cytopathology in Focus: Pap proficiency testing: what’s permitted, what’s not
January 2018—Annual proficiency testing is mandated for all cytotechnologists and pathologists who sign out Pap tests. CAP staff frequently receive questions about the PT rules or hear of situations in which there was confusion about the rules, a few of which we will highlight here.
Read More »Cytopathology in Focus: HPV vaccines: the decade in review
January 2018—Diane Harper, MD, MPH, and Leslie DeMars, MD, provide an extensive review of the efficacy of available FDA-approved HPV vaccines in different age groups and describe immunogenicity findings in particular (Gynecol Oncol. 2017;146:196–204). The World Health Organization and the Centers for Disease Control and Prevention recommend a two-dose vaccine for younger children due to high rates of seroconversion and antibody titers in this age group. Girls age 15 and older should continue to get three doses.
Read More »Anatomic Pathology Abstracts, 1/18
January 2018—Analysis of desmoplastic pattern at the tumor front in colorectal cancer subtypes: Although recent findings of cancer biology research indicate that prognostic power arises from genes expressed by stromal cells rather than epithelial cells, desmoplastic reaction has not been completely examined as a prognostic marker for colorectal cancer. The authors conducted a pathologic review of 821 stage II and III patients who underwent R0 resection for colorectal cancer at four independent institutions.
Read More »Clinical Pathology Abstracts, 1/18
January 2018—Drone transport of chemistry and hematology samples over long distances: Interest in using unmanned aerial vehicles, or drones, to transport laboratory specimens is based on the need to move specimens from satellite facilities to a central hub for testing. Earlier studies of biological specimens transported by drones were performed in ambient or cold temperatures for a maximum flight length of 40 minutes.
Read More »Molecular Pathology Abstracts, 1/18
January 2018—Importance of interstitial genes that exist between gene fusion partners in prostate cancer: Prostate cancer is one of the most common cancers affecting men, yet understanding of the disease’s development and progression is limited. One of the most effective ways to stratify treatment and outcomes is based on pathology review of prostate biopsies, though the application of molecular testing to these samples is increasing.
Read More »Newsbytes, 1/18
January 2018—Why pathologists shouldn’t ‘pass the baton’ with IT: It may be tempting to stay in your comfort zone and leave the technology decisions to the information technology experts. But pathologists who abdicate oversight of IT projects within their departments are setting up those projects for failure, says John H. Sinard, MD, PhD, professor of pathology and medical director of pathology informatics at Yale University School of Medicine.
Read More »Q&A column, 1/18
January 2018—We are in the process of validating the Stago STA Compact Max and Stago STA R Max with cap piercing. The company is stating that the open and closed modes follow the same testing pathway and therefore validation between modes is not necessary. Is this correct? Is PHI (phosphohexose isomerase), also known as GPI (glucose phosphate isomerase), mainly responsible for metastasis and circulating tumor cells?
Read More »Put It on the Board, 1/18
January 2018—Some safety issues more gray than black and white: Most laboratory safety rules are free from ambiguity. Anyone who handles specimens must wear personal protective equipment, for example. Some issues are less clearly defined, though, and require a deeper dive into the guidelines. That is where Dan Scungio, MT (ASCP), SLS, CQA(ASQ), comes in.
Read More »Hopes, fears as users switch to new troponin
December 2017—The questions that arise over the use of highly sensitive cardiac troponin are as riveting as, if less historically fraught than, the Jefferson-Hamilton debates over the shape of their newborn country. Who should lead—the states or a strong central government? Cardiologists or the emergency department? What cutoffs represent the right balance between admissions, referrals, and sending patients home? And will Lin-Manuel Miranda ever write a smash musical about this cardiac assay?
Read More »In hemostasis, two hot-button testing issues
December 2017—Having validation data to support the use of age-adjusted D-dimer cutoffs with the D-dimer assay your laboratory uses is a must, and know well the limitations of point-of-care prothrombin time/INR testing. That advice and more was shared in a “Hot Topics in Hemostasis” session at CAP17, presented by Russell Higgins, MD, and Karen Moser, MD.
Read More »‘Connectathon’ opens door to interoperability in digital pathology
December 2017—With the FDA having approved whole slide imaging for primary diagnosis this year, one obstacle to full acceptance of digital pathology remains: lack of interoperability. To topple that barrier, the Digital Pathology Association, the CAP through its Digital Pathology Committee, and DICOM Working Group 26 convened in October, during the Pathology Visions conference, the first Connectathon for digital pathology.
Read More »HbA1c shows its mettle in predicting diabetes risk
December 2017—The longitudinal Framingham Heart Study, which first identified the concept of risk factors and made serum LDL cholesterol a household name, could help increase the celebrity status of HbA1c, with the Oct. 26 publication of a new study in Diabetes Care. International and national organizations since 2010 have recognized HbA1c as a valid way to diagnose abnormalities in glycemia and diabetes mellitus. But there has been less consensus on its use as a screen for elevated diabetes risk.
Read More »From the President’s Desk: Family matters, 12/17
December 2017—Most of us look forward to the holidays. We are excited about renewing old connections or making new ones, getting together with family and friends, having special meals, attending comforting services, gathering together to exchange gifts or just talk. We look forward to the comforts of predictability. The corny Dad jokes that must be retold. Family stories, those true and nearly true, are relived together and, for the younger members of the family, may be heard for the first time. Familiar, treasured television shows are seen for the umpteenth time. Beautiful lights, shows, pageants, and parades dazzle all, young and old.
Read More »Higher pay for therapeutic apheresis, bone marrow aspiration
December 2017—For 2018, CMS estimates a one percent overall decrease in pathology reimbursement. Pathologists will receive payment increases for therapeutic apheresis and diagnostic bone marrow aspiration services in 2018. At the same time, reimbursement for flow cytometry services will continue to decrease following phased-in reductions set by the Medicare program last year, but the CAP was successful in lessening the impact of cuts to those services in 2018.
Read More »Automation, standardization lead the way in urinalysis
December 2017—Smaller-scale technology and standardization are just some of what laboratories need, and it’s where the companies that make urinalysis analyzers are in part focusing their work. CAP Today spoke with three of the five whose analyzers are profiled on the following pages.
Read More »Targeted NGS or exome? Consider the clinical context
December 2017—American writer Maile Meloy published a short story collection in 2009 titled Both Ways Is the Only Way I Want It. Molecular pathology laboratory directors faced with the variety of next-generation sequencing diagnostic panels might feel similarly. As the main character in Meloy’s title story asks, “What kind of fool wanted it only one way?”
Read More »Are point-of-care PT/INR devices safe and effective?
December 2017—Safety issues related to point-of-care PT/INR testing surfaced in recent years, among them a 2016 voluntary class 1 recall of Alere’s INRatio and INRatio2 monitor systems. “Prior to that, the company that manufactured the device had received thousands of complaints about it,” says Russell Higgins, MD, of the University of Texas Health Science Center at San Antonio.
Read More »Class act in Ohio expands pool of phlebotomists
December 2017—After two rounds of a new program to train high schoolers in phlebotomy, OhioHealth is seeing the fruits of its efforts. It has hired 19 of its trainees and a third course, set to begin next month, has 20 high school seniors enrolled. Just when OhioHealth’s phlebotomy staffing needs were expanding, laboratory services leaders were growing increasingly dissatisfied with the quality of the training students were receiving at most of the phlebotomy programs in the Columbus area.
Read More »Anatomic Pathology Abstracts, 12/17
December 2017—Gene mutations in HPV-negative penile squamous cell carcinoma: The majority of penile squamous cell carcinomas are caused by transforming human papillomavirus infection. The etiology of HPV-negative cancers is unclear, but TP53 mutations have been implicated. Archival tissue from 108 invasive squamous cell carcinomas from a single pathology institution in a low-incidence area were analyzed for HPV-DNA and p16INK4A overexpression and for TP53 mutations by Ion Torrent next-generation sequencing.
Read More »Clinical Pathology Abstracts, 12/17
December 2017—Chimeric antigen receptor T-cell therapy assessment and management of toxicities: In August, the FDA approved the gene therapy Kymriah (tisagenlecleucel) for pediatric and young adult patients with a form of acute lymphoblastic leukemia. Chimeric antigen receptor (CAR) T-cell therapy is a breakthrough in the treatment of leukemia and lymphoma, but it is associated with unique acute toxicities, such as cytokine-release syndrome and CAR-T-cell-related encephalopathy syndrome.
Read More »Molecular Pathology Abstracts, 12/17
December 2017—Refining subgroups of pediatric gliomas using molecular markers: Pediatric high-grade and diffuse intrinsic pontine gliomas are a rare and heterogeneous group of tumors that show diverse histology, location, and prognosis. Although little was known regarding the development of these tumors, recent genomic studies have begun to elucidate their biological underpinnings. The authors conducted a study to further understanding of such gliomas by breaking them into subgroups.
Read More »Q&A column, 12/17
December 2017—Is a tumor embolus in the capsule of the lymph nodes considered metastasis? Does the lung, like the lymph nodes of the breast, have the concept of isolated tumor cells? As the staging rule is “when in doubt, understage,” would the case therefore be pT2, N0?
Read More »Newsbytes, 12/17
December 2017—Hospital cyberattack a brief setback with lasting gain: A cyberattack that paralyzed the computer systems at a rural West Virginia hospital last summer could have brought the laboratory’s work to a screeching halt. But that didn’t happen, thanks, in part, to the downtime procedures in place throughout the laboratory and the low-tech nature of the lab’s pathology operations.
Read More »Put It on the Board, 12/17
December 2017—FDA announces IMPACT authorization and path for authorization of other tumor profiling tests: The FDA on Nov. 15 authorized Memorial Sloan Kettering Cancer Center’s IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets) tumor profiling test. The IMPACT test uses next-generation sequencing to identify the presence of mutations in 468 unique genes, as well as other molecular changes in the genomic makeup of a tumor.
Read More »AMP case report: November 2017 test yourself answers
December 2017—In the November 2017 issue was a report (page 26), “Follicular lymphoma of gallbladder: Use of immunoglobulin gene clonality studies to facilitate diagnosis of unusual case,” written by members of the Association for Molecular Pathology. Here are answers (in bold) to the three “test yourself ” questions that followed that case report.
Read More »Next-gen troponin: out of the gate, into labs
November 2017—The story of highly sensitive cardiac troponin, as written by Dr. Seuss, would provide a small twist. In this version, the Grinch doesn’t steal Christmas. Rather, he keeps delaying it, quarter after quarter, year after year. “I remember maybe seven years ago, Roche told me their assay was coming. It’s coming, it’s coming, it’s coming,” laughs Sihe Wang, PhD, medical director and section head, clinical biochemistry, Cleveland Clinic, and clinical chemistry professor, Cleveland State University.
Read More »Benefits and bumps of shifting to Beaker
November 2017—If they were located in the Land of Oz, laboratories selecting a laboratory information system might not have to make a choice between full functionality and seamless integration with their electronic medical record system. They could just follow the helpful advice of the Scarecrow to Dorothy at a crossroads: “Go both ways.”
Read More »LIS niche modules flourish amid IT consolidation
November 2017—“There’s an app for that” was a common, if flippant, catch phrase to suggest that a software solution had already been devised for just about every need (at least until 2010, when Apple trademarked the catch phrase). In the laboratory industry today, you are likely to hear more references to software’s “functionality,” but the concept is the same. While debate continues over whether best-of-breed products or comprehensive information technology systems should rule the laboratory, health care IT companies have developed a profusion of modules or ancillary applications—sometimes packaged with an LIS, sometimes sold separately—to fill software gaps.
Read More »With syphilis rates rising sharply, syphilis tests a focus
November 2017—Syphilis is making a comeback. Nearly 28,000 cases of primary and secondary syphilis, the most infectious stages of the disease, were reported in the U.S. in 2016—a 17.6 percent jump over 2015 and the highest reported rate since 1993. Cumbersome, subjective nontreponemal assays and the lack of a gold standard screening method lend complexity to the diagnostic process. But new nontreponemal assay options, including the first FDA-cleared fully automated treponemal/nontreponemal dual assay, may help stem the rising tide.
Read More »From the President’s Desk: CAP17—No match for being there, 11/17
November 2017—My sister Jean had a Chatty Cathy doll; you pulled a cord in the back of her neck and she would say one of a handful of things (“Would you like some tea?”). Chatty Cathy was Jean’s favorite for a while, supplanting her much-loved Raggedy Ann. The coup lasted just long enough to make it plain that the new kid on the block didn’t have much to say for herself. She was kind of stiff and controlling, really. Raggedy Ann was a much better listener. Making up stories with her was a lot more fun.
Read More »New sections added to AP, cytopathology checklists
November 2017—A new flow cytometry section in the anatomic pathology checklist and a section on immunochemistry in the cytopathology checklist are among the many changes found in the latest edition of the CAP Laboratory Accreditation Program checklists, released in August.
Read More »Diagnostic teams: five barriers but the time is now
November 2017—Many people have case conferences, but a true diagnostic management team is one in which four things happen. First, you have to meet frequently and regularly, and you have to provide a patient-specific report. Second, the report must be delivered before or during the time when treatment decisions are made. This is why a once-a-month meeting doesn’t work.
Read More »AMP case report: Follicular lymphoma of gallbladder, November 2017
November 2017—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Western University and London Health Sciences Centre, London, Ontario, Canada. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Read More »Clinical Pathology Abstracts, 11/17
November 2017—Performance of virological testing for early infant diagnosis: a systematic review: The World Health Organization recommends that HIV-exposed infants receive virological testing for HIV infection between four and six weeks of age and treatment with antiretroviral (ARV) therapy as soon as the diagnosis is made. Despite efforts to expand mother-to-child transmission prevention programs, only an estimated 50 percent of HIV-exposed infants are tested within the first two months of life.
Read More »Anatomic Pathology Abstracts, 11/17
November 2017—MCM2: an alternative to Ki-67 for measuring breast cancer cell proliferatio: Breast cancer is a heterogeneous disease comprised of a diversity of tumor subtypes that manifest themselves in a wide variety of clinical, pathological, and molecular features.
Read More »Molecular Pathology Abstracts, 11/17
November 2017—Molecular analysis of colorectal tumors in Lynch syndrome: Lynch syndrome, most often caused by a germline mutation in MSH2, MSH6, MLH1, or PMS2, contributes to a number of malignancies, including colorectal and endometrial cancer.
Read More »Newsbytes, 11/17
November 2017—Software-validation products: finding a glitch before it’s a hitch: The idiom “time is of the essence” isn’t lost on Lisa Adams, senior information technology systems analyst at Banner Health and a believer in the need for speed when identifying software glitches and errors.
Read More »Q&A column, 11/17
November 2017—A laboratory owns chemistry analyzers from company X. Company X recommends that its customers use company X’s calibration material to perform their linearity studies, starting with the highest concentration and using the chemistry analyzer’s autodilution feature to provide a total of four measurable concentrations and a final zero point. Does this protocol fulfill CAP checklist requirements?
Read More »Put It on the Board, 11/17
November 2017—Study finds biotin interference; FDA clears Flu A/B/RSV assay on Hologic’s Panther Fusion; FDA clears Abbott’s Alinity ci-series; Sysmex introduces CyFlow Antibodies for flow cytometry; Test approved for screening Zika in blood donations; New insights into female reproductive tract development
Read More »Letters, 11/17
Biotin pharmacokinetic study results: In Anne Paxton’s September 2016 article, “Beauty fad’s ugly downside: test interference,” I stated a commitment from Roche to reduce possible interferences, including biotin, and provide clear test labeling to ensure that physicians and laboratories can mitigate risk.
Read More »AMP case report: October 2017 test yourself answers
November 2017—In the October 2017 issue was a report, “Primary pulmonary adenocarcinoma with an unusual molecular profile of the EGFR gene at initial presentation,” written by members of the Association for Molecular Pathology. Here are answers (in bold) to the three “test yourself ” questions that followed that case report.
Read More »‘Split’ decisions in CNS tumor update
October 2017—Classifying central nervous system tumors has recently become both more complex and easier. Surgical pathologists now have guidance that helps them work through the whys, hows, and what-ifs of using molecular studies when making diagnoses. The 2016 WHO classification for CNS tumors, which has been described as a conceptual and practical advance over the previous incarnation, from 2007, should also help them move closer to precision medicine.
Read More »Revived hopes, fresh challenges with liquid biopsy
October 2017—Until recently, new treatments for stage 4 lung cancer have generally required weighing toxicity against hopes that patients’ average length of survival might be extended by a month or two. But “our expectations are increasing as therapies have improved,” says Geoff Oxnard, MD, thoracic oncologist at Dana-Farber Cancer Institute and assistant professor of medicine at Harvard Medical School. “Patients and doctors are increasingly expecting targeted therapies with dramatic effect and few side effects.”
Read More »PD-L1 guideline panels hustle to keep pace with drug advances
October 2017—The expert and advisory panels for the CAP/IASLC/AMP guideline on molecular testing for lung cancer biomarkers started updating the guideline in 2014, and an important but fairly routine revision process may have seemed to lie ahead. Something like sedately stepping onto a moving sidewalk. The key question at that point was quotidian: Have new data emerged to warrant changing the original recommendations?
Read More »How to spot the savings from a diagnostic team
October 2017—Few pathologists and laboratory professionals would argue with the potential clinical benefit of a diagnostic management team, a group that meets often and provides timely patient-specific reports that synthesize all test results. But getting C-suite executives on board may mean uncovering whether such a team can save the hospital money.
Read More »From the President’s Desk: Let us make no small plans, 10/17
October 2017—I understand that you’re reading this several weeks after CAP17, after I’ve been sworn in, after we’ve returned home thinking about friendships renewed, controversies debated, new tools encountered up close. Although I write this before the meeting, I know how I will feel by the time you read it: grateful and glad to be a member of this organization.
Read More »Recommendations for investigating liver chemistry abnormalities are unworkable
October 2017—A new guideline on the evaluation of abnormal liver chemistries was published in the January 2017 issue of the American Journal of Gastroenterology (AJG).1 The guideline, developed by the American College of Gastroenterology’s practice parameters committee, is based on three resources, the first of which is a review of published research. The other two resources are notably similar and largely based in expert opinion.
Read More »DIY or Survey? Identifying interfering substances
October 2017—The interfering substance: Whether it’s in-laws on your doorstep or lipemia in your specimen, it has to be addressed. Ask Michelle K. Zimmerman, MD. These days, Dr. Zimmerman uses the CAP Interfering Substance Survey to detect the presence of hemolysis, lipemia, and icterus in clinical chemistry samples at Indiana University School of Medicine, where she is an assistant professor of pathology and laboratory medicine. But before her laboratory started using the Survey, how did it handle those interferences?
Read More »AMP case report: Primary pulmonary adenocarcinoma with an unusual molecular profile of the EGFR gene at initial presentation, October 2017
October 2017—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from the University of Massachusetts Medical School-Baystate, Springfield. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Read More »NGS checklist takes in infectious disease testing
October 2017—The CAP issued its first accreditation checklist for next-generation sequencing in 2014, as NGS was becoming a tool used in a growing number of clinical laboratories. The list of requirements, which was a new section in the molecular pathology checklist, focused on constitutive (germline) testing and oncology testing.
Read More »Sigma analysis, role and limitations: development of a QC program for the Beckman Coulter AU5812
October 2017—The challenge for all clinical laboratories is to produce the highest quality in vitro diagnostic results in the most efficient manner. Fortunately, high quality and high efficiency are not mutually exclusive, and the direct correlation between the two is well documented.1,2 As the quality of processes increases, so does process efficiency, which ultimately drives down costs.
Read More »Clinical Pathology Abstracts, 10/17
October 2017—Association between age at natural menopause and risk of type 2 diabetes: Menopause in women marks the loss of ovarian follicle development and the timing of the final menstrual period. The timing of menopause differs significantly among women and is seen as a marker of aging and cardiovascular health. Studies have shown a link between early onset of menopause and an increased risk of cardiovascular disease (CVD) and overall mortality, whereas menopause at age 50 to 54 years is linked to a decrease in CVD risk and mortality.
Read More »Anatomic Pathology Abstracts, 10/17
October 2017—Lymph node yield is an independent predictor of survival in rectal cancer: Lymph node yield is used as a marker of adequate oncological resection. The American Joint Committee on Cancer recommends at least 12 nodes to confirm node-negative disease for rectal cancer. However, it is not always possible to achieve a lymph node yield of 12, particularly in patients who have undergone neoadjuvant treatment.
Read More »Molecular Pathology Abstracts, 10/17
Editors: Donna E. Hansel, MD, PhD, chief, Division of Anatomic Pathology, and professor, Department of Pathology, University of California, San Diego; John A. Thorson, MD, PhD, associate professor of pathology, director of the Clinical Genomics Laboratory, Center for Advanced Laboratory Medicine, UCSD; Sarah S. Murray, PhD, associate professor, Department of Pathology, and director of genomic technologies, Center for Advanced Laboratory ...
Read More »Q&A column, 10/17
October 2017—Our doctors request strep group A culture on throat specimens that are negative for rapid strep group A. On culture workup, if we have beta-hemolytic strep, we perform latex grouping only for group A strep; we report negative for GAS if latex is negative and positive if latex is positive. I think we should confirm all GAS with pyrrolidonyl arylamidase (PYR), and group and report other non-GAS. What do you think?
Read More »Newsbytes, 10/17
October 2017—Clinical analytics: from benefits attained to software available: While LIS and laboratory billing software vendors tout the power of their business analytics tools to boost the laboratory’s bottom line, a newer application of information technology—clinical analytics—is elevating the role of the laboratory in personalized medicine.
Read More »Put It on the Board, 10/17
October 2017—Houston labs learn: know the back roads: The disaster plan of the laboratories at Memorial Hermann Health System in Houston held up well in Hurricane Harvey, thanks to lessons learned in years past, but the labs have something new to add: Know in advance the back-road access routes to the various hospitals.
Read More »Letters, 10/17
CAP TODAY provides valuable information that is important to guide the practice for many pathologists. However, an article published in the August 2017 issue, “Laboratory director duties clarified in 2017 checklist” by Anne Ford, has raised serious concerns among many pathologists, particularly members of the Chinese American Pathologists Association (CAPA).
Read More »New tests, new wrinkles in HIV algorithm
September 2017—Three years—including a total eclipse of the sun—have sped by since the Centers for Disease Control and Prevention and the Association of Public Health Laboratories recommended a new HIV diagnostic testing algorithm for laboratories. In 2014, the algorithm was seen as bringing HIV test ordering up to speed with the advances in HIV test technology and increasing the accuracy and reliability of HIV screening and diagnosis. Have laboratories made the adjustment, and is the CDC/APHL algorithm proving workable and worthwhile?
Read More »Turning points in transgender medicine
September 2017—The intricacies of transgender medicine are many. They are unique; they are universal. A la Walt Whitman, they contain multitudes: identities, challenges, questions, even fears. But the first step toward comprehending them can be simple. Tim Cavanaugh, MD, started with a cup of coffee. Dr. Cavanaugh, of Fenway Health, Boston, began delving into the topic about a decade ago, when an assistant administrator at his previous job, at a small community health center in Rhode Island, told the center’s leaders that the transgender population was medically underserved.
Read More »Advanced parameters offer faster, surer guidance to cancer care
September 2017—After a career spent studying malignancies in the bone marrow and monitoring the effects of chemotherapy on the bone marrow and neoplastic cells contained therein, Cheryl Hirsch-Ginsberg, MD, stepped out from the bone marrow realm and into the faster-paced world of high-volume hematology.
Read More »AMP case report: August 2017 test yourself answers
In the August 2017 issue was a report, “NGS panel aids in diagnosis of rare collision tumor,” written by members of the Association for Molecular Pathology. Here are answers (in bold) to the three “test yourself ” questions that followed that case report.
Read More »Market based? A view of PAMA process, pricing
September 2017—Under the Protecting Access to Medicare Act of 2014, Medicare rates for laboratory tests will be recalculated to reflect “market-based pricing” as reported by “applicable laboratories.” But are labs going to get a market-based price, or is the Centers for Medicare and Medicaid Services “gaming the system to ensure there will be a cut to the fee schedule”?
Read More »From the President’s Desk: Entrustable professional activities, 9/17
September 2017—Academic Pathology recently published the results of a two-year project by the CAP Graduate Medical Education Committee to build a framework for teaching specific entrustable professional activities (EPAs) in pathology. Their scheme structures a competency-based approach to training mapped to the ACGME Milestones for pathology resident evaluation. Competency-based curricula are well suited to impart the breadth and depth of necessary fundamental knowledge to future pathologists.
Read More »New requirement, updates in transfusion checklist
September 2017—Like an old friend with a new facelift, or a high-mileage car with a thorough tune-up, the 2017 edition of the CAP transfusion medicine checklist has undergone a significant number of small changes—none of which is startling in itself, but all of which combine to produce a fresh and streamlined effect. More than 90 of the checklist’s requirements have been revised, many in the name of alignment with FDA requirements.
Read More »Antibiotic stewardship gets a boost in FDA’s OK
September 2017—Those who have seen the strength of antibiotic stewardship hope the FDA’s clearance this year of an assay for antibiotic management will drive further and move faster the efforts being made to curb unnecessary use.
Read More »Bringing data analytics to bear on diabetes care
September 2017—Can data move the dial on diabetes? That’s the thinking behind Roche Diabetes Care’s new partnership with Accenture, and it’s how some labs and health care systems are already driving diabetes care to a whole new level.
Read More »For NIPT laboratories, a new proficiency test in 2018
September 2017—The CAP Surveys program is offering for 2018 a novel external proficiency test for laboratories that perform screening of cell-free (cf) DNA in maternal plasma for common aneuploidies.
Read More »For Quality Registry, details and demos at CAP17
September 2017—The CAP is set to launch next month the Pathologists Quality Registry for pathologists to begin using in 2018 to collect data under Medicare’s Quality Payment Program (QPP) Merit-based Incentive Payment System (MIPS) track.
Read More »Clinical Pathology Abstracts, 9/17
September 2017—Hemoccult testing before therapeutic anticoagulation in venous thromboembolism: Gastrointestinal bleeding is a major adverse event associated with therapeutic anticoagulation. Surveys of physicians have shown that concern for this event is one of the most common reasons to withhold anticoagulation in patients who have atrial fibrillation, acute coronary syndromes, or venous thromboembolism (VTE).
Read More »Anatomic Pathology Abstracts, 9/17
Editors: Michael Cibull, MD, professor emeritus, University of Kentucky College of Medicine, Lexington; Rouzan Karabakhtsian, MD, PhD, associate professor of pathology, Albert Einstein College of Medicine, Montefiore Medical Center, Bronx, NY; Thomas Cibull, MD, dermatopathologist, Evanston Hospital, NorthShore University HealthSystem, Evanston, Ill.; and Rachel Stewart, DO, resident physician, Department of Pathology and Laboratory Medicine, University of Kentucky. Detection of HPV ...
Read More »Molecular Pathology Abstracts, 9/17
September 2017—Ability of cell-free circulating tumor DNA to reflect genomic changes in cancer deposits: Analysis of cell-free circulating tumor DNA is an emerging precision medicine technology that may be used to assess molecular alterations in cancer-derived DNA present in the blood, as well as to monitor cancer genomic changes over time and assess genomic changes and resistance following cancer therapy.
Read More »Letters, 9/17
September 2017—I write this after reading “Total joints in view: to tilt at or to toss” (July 2017). I just completed my 42nd year as a general pathologist in an acute care community hospital that had 100 beds in 1971 and now has close to 500. From about 1980 (before we went to “separate billing”) through 2014, I fought relentlessly to have and keep the policy that all tissue get a pathology exam. These exams are needed:
Read More »Q&A column, 9/17
September 2017— I received a sample with very high hemoglobin grossly. When I ran the sample on the Cell-Dyn Ruby, it was unable to calculate the parameters related to Hgb. I diluted the EDTA blood and ran the test again. In this scenario, should I multiply all the indices and Hgb-related parameters with the dilution factor? Which parameters should I multiply with the dilution factor?
Read More »Newsbytes, 9/17
September 2017—Why so few women in pathology informatics? Alexis Carter, MD, did not realize she was a rare bird when, as a resident more than a decade ago, she acted on her penchant for health informatics. Dr. Carter had become interested in the field while working under a clinical chemist who developed computer programs that notified him when instruments weren’t performing as expected or when a lab result required further investigation.
Read More »Put It on the Board, 9/17
September 2017—Eliminating CK-MB testing in suspected ACS: Health care leaders and clinicians should design and implement hospital-wide educational campaigns and partner with information technology and/or laboratory medicine staff at their institutions to remove CK-MB from standardized acute coronary syndrome routine order sets, say authors of a blueprint that could be a “first step to finally putting the CK-MB laboratory test to rest.”
Read More »Making it personal: transgender medicine
August 2017—Talk about personalized medicine. While the national discussion about transgender women and men often pivots on civil rights legislation (exhibit A: so-called bathroom bills), the medical community has quietly begun to ask questions about how to provide care for transgender patients. In the process, assumptions are being turned sideways. And as laboratory professionals are realizing, the impact can affect everything from start (patient identification, test ordering) to finish (test results, billing), seemingly one patient at a time.
Read More »A slimmer molecular micro section among changes to checklists
August 2017—There was no trip to the spa. But some sections of the 2017 edition of the CAP Laboratory Accreditation Program checklist are looking trimmed and toned compared with last year’s checklists. A microbiology section that is shorter by eight pages, fewer Individualized Quality Control Plan reporting requirements, and a new section addressing chain of custody once again reflect the hard work of the Checklists Committee and scientific resource committees to achieve conciseness and clarity.
Read More »In digital age, new focus on specimen, slide prep
August 2017—The age of FDA-approved whole slide imaging for primary diagnosis has dawned with opportunity for every level of professional who works in the digital pathology environment. It includes not only an expanded professional cachet but also great potential born of collaborative and remote capabilities, and perhaps better patient outcomes as a result.
Read More »From the President’s Desk: Staying close to our knitting, 8/17
August 2017—Nearly two years ago, I mentioned that I wanted to start a conversation about how we as pathologists and the CAP as our professional society must evolve in order to meet our emerging needs as individuals and as a specialty. Now, as my time in the perch is coming to a close, I’d like to explore what we have come to realize—and sometimes reinforced—about building and maintaining a complex infrastructure that reflects and serves our core purposes.
Read More »Ownership remix as hospitals, national labs jockey for position
August 2017—In the game of Risk, dominating the board hinges not only on clever strategy but also on rolls of the dice. In the real-world game that is the enormous laboratory market, there is a parallel: Rationally calculating the profitability and risk of mergers or acquisitions is crucial, but many such business moves involve a gamble. Right now, the main thing the laboratory industry appears to be betting on is upheaval.
Read More »AMP case report: NGS panel aids in diagnosis of rare collision tumor, August 2017
August 2017—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Columbia University Medical Center. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Read More »Laboratory director duties clarified in 2017 checklist
August 2017—Quantum theory is often interpreted to mean an object can be in two places simultaneously. Unfortunately, quantum theory doesn’t apply to laboratory directors, at least not on a scheduling level. Like the rest of us, directors can be in only one place at a time, no matter how many laboratories they oversee. Now a change to the CAP Laboratory Accreditation Program’s checklists will clarify expectations for directors who are in charge of more than one laboratory. The 2017 edition of the checklists, released this month, has eliminated the specific requirements for laboratory directors who are not on site full time and has clarified responsibilities for all directors, on site or remote.
Read More »Cytopathology in Focus: Cell Blocks: Getting the most from the least invasive method
August 2017—Adequate and high-quality cell block preparations can be a useful adjunct to cytologic smear preparations and touch imprint cytology. Adequate cell blocks allow for additional studies and can provide a specific diagnosis and information essential for targeted treatment plans. Cell blocks can be prepared from most cytology specimens such as fine needle aspirations, body cavity fluids, washings, brushings, and gynecologic and nongynecologic liquid-based specimens.
Read More »Cytopathology in Focus: Closing cytopathology, cytotechnology practice gaps—three years later
August 2017—The CAP has focused during the past 10 years on facilitating the transformation of pathology practices from the instrumentation age to the information age, with a concentration on personalized medical care and laboratorians as integral members of the health care team. Hand-in-hand with that effort, the CAP Cytopathology Committee has advocated for the expanded use of cytology specimens in molecular diagnostics and the evolution of the cytotechnology workforce to meet the emerging practice gaps as pathologists become engaged in ever more complex diagnostic processes.
Read More »Molecular Pathology Abstracts, 8/17
August 2017—Mismatch repair-deficient tumors and immune checkpoint inhibitors: Immune checkpoint inhibitors have yielded highly effective therapeutic responses in a subset of tumors by eliciting an endogenous adaptive immune response. The determinants that define this subset of tumors are still unclear, but several markers, including PD-L1 expression and mutational burden, have been evaluated in various tumor types.
Read More »Anatomic Pathology Abstracts, 8/17
August 2017—Analysis of microglandular adenosis and acinic cell carcinoma of the breast: Acinic cell carcinoma is an indolent form of invasive breast cancer, whereas microglandular adenosis has been shown to be a neoplastic proliferation. Both entities display a triple-negative phenotype and may give rise to, as well as display, somatic genomic alterations typical of high-grade triple-negative breast cancers. The authors compared previously published data on eight carcinoma-associated microglandular adenoses and eight acinic cell carcinomas subjected to massively parallel sequencing targeting all exons of 236 genes recurrently mutated in breast cancer or DNA repair related, or both.
Read More »Clinical Pathology Abstracts, 8/17
August 2017—Etiology and clinical presentation of birth defects: a population-based study: Birth defects are inborn errors of development and include any structural or functional anomaly that impacts physical, intellectual, or social well-being. They are a considerable and growing clinical and public health challenge. Major birth defects are common and costly. Collectively, they are estimated to occur in one in 33 births, which translates into approximately 7.9 million babies affected worldwide. In the United States alone, the cost of care during a single year (2004) was estimated to be $2.6 billion.
Read More »Q&A column, 8/17
August 2017—Due to an ever-changing workforce, many new and inexperienced technologists are working in the microbiology lab and appear to be having difficulty interpreting cultures and troubleshooting when an organism in question may not be significant. As an example, a scant growth of Micrococcus was isolated and reported from a cerebrospinal fluid culture; it was not seen in the Gram stain and was negative for leukocytes. Contaminants had been noted on some of the media plates at this time as well, but many of these inexperienced technologists do not have the confidence to ignore obvious contaminants or suggest the possibility of contamination. Is there some guidance or troubleshooting tools for these situations?
Read More »Newsbytes, 8/17
August 2017—Health record security at root of personal grid architecture: Imagine the risks credit reporting agencies would face if they did not maintain databases of consumer transactions but instead requested information from various creditors and assembled credit reports from that information in real time. Yet that’s how health information exchanges typically work. And that, says William Yasnoff, MD, PhD, a consultant, physician, and computer scientist, is not a safe or effective approach.
Read More »Put It on the Board, 8/17
August 2017—AML drug approved with companion diagnostic: The Food and Drug Administration approved Idhifa (enasidenib) for the treatment of adult patients with relapsed or refractory acute myeloid leukemia who have a specific genetic mutation. The drug is approved for use with the RealTime IDH2 Assay, which is used to detect specific mutations in the IDH2 gene in patients with AML.
Read More »Total joints in view: to tilt at or to toss
July 2017—One of the more unnerving scenes in contemporary theater comes courtesy of Martin McDonagh’s “A Skull in Connemara,” which opens with two men in an Irish graveyard, hired by the local priest to make room in the overcrowded burial ground. Their method? Exhume the corpses and smash the bones to bits.
Read More »Outreach: Forge ahead or accept purchase bid?
July 2017—With the laboratory industry in flux—and many critical determinants of the next few years waiting on policy moves by the new administration and third-party payers—hospital outreach programs could wish for a better time to make existential decisions such as accepting an offer to be purchased.
Read More »Volume, value, technology steering 2017 instrument buys
July 2017—For at least some laboratories, economic conditions and capital flows are calling for a cautious approach to purchasing new laboratory instruments. As one analyst of the clinical laboratory services industry was heard to say recently: “Because of tight capital, nobody is buying anything unless it breaks.” But laboratory executives and medical directors at some of the nation’s largest health systems in the Northeast, West, and Midwest take a different view.
Read More »With diversion, lower blood culture contamination rates
July 2017—To stage magicians, diversion is a trick—a way to direct the audience’s attention to something irrelevant so they don’t notice what they shouldn’t see. To those who perform blood cultures, diversion is also a trick, though there’s nothing deceptive about it—and the way it helps avoid contamination can seem like magic.
Read More »From the President’s Desk: Is ‘breaking news’ breaking ‘news’?
July 2017—Once upon a time, “breaking news” referred to critical information on the order of an assassination or act of war. Over time, its use came to embrace emergencies and disasters. Now, it seems that anything more curious than “man bites dog” qualifies.
Read More »Hepatic neoplasms—cases, challenges, cautions
July 2017—Kisha Mitchell Richards, MBBS, once took a picture of the ocean as she went around a bend in the road traveling from Negril to Montego Bay in Jamaica. She showed that photo in the second half of a CAP16 session to prepare the audience to shift gears, as she put it, from the first speaker’s talk on medical liver disease (see “Liver injury patterns: pitfalls and pointers,” March 2017) to hers on hepatic neoplasms. “So for me, we are about to go around a bend to things of sheer beauty,” she said, referring to immunohistochemistry stains in the neoplastic liver. “Unfortunately, that which is beautiful to the pathologist is not often great for the patient. That’s our usual practice,” said Dr. Richards, a pathologist at Greenwich Hospital, Yale New Haven Health, Greenwich, Conn.
Read More »Hepatocellular adenoma subtypes—Which is it?
July 2017—Kisha Mitchell Richards, MBBS, a pathologist at Greenwich Hospital, Yale New Haven Health, Greenwich, Conn., recalls that when she was a resident, adenoma was just adenoma. “Nowadays it’s not quite where breast is, where it’s a two-page report, but there are now subtypes of hepatocellular adenomas,” she said in a CAP16 presentation on liver neoplasms. The subtypes are the HNF1 alpha or TCF1 inactivated adenoma, inflammatory adenoma, beta-catenin mutated adenoma, and the unclassified adenoma, which she notes is basically adenoma NOS (not otherwise specified).
Read More »Integrative consults remove referral inefficiencies
July 2017—Inappropriate referrals to rheumatologists and months-long wait times led pathologists to start a service at Harris Health in Houston of consultative-algorithmic workups for rheumatologic disease. “Everyone liked it. Rheumatologists were happy to get patients they could treat and who were already worked up,” Robert L. Hunter, MD, PhD, says of the service that gave primary care providers the option of selecting algorithmic testing with pathologist consultation rather than order individual tests when signs and symptoms suggested rheumatologic disease.
Read More »New pathology patient consult program takes off
July 2017—Ten weeks in, 10 patients seen. The pathology patient consult program at Lowell General Hospital is already giving a boost to pathology’s visibility and patients a better understanding of their disease. Lija Joseph, MD, chief of pathology and medical director of pathology and laboratory medicine at Lowell General Hospital in Lowell, Mass., says a colleague’s presentation about social media and medicine led her to launch the patient pathology consult program in March.
Read More »Multiplexed inherited cancer reference material, 7/17
July 2017—SeraCare Life Sciences launched the industry’s first multiplexed inherited cancer reference material for inherited disease testing by next-generation sequencing, according to the company. The Seraseq Inherited Cancer DNA Mix reference materials were developed to validate the ability of synthetic reference materials to address technically challenging variants.
Read More »15th phlebotomy edition holds ‘latest, greatest’
July 2017—After overseeing 10 editions of So You’re Going to Collect a Blood Specimen: An Introduction to Phlebotomy, Frederick L. Kiechle, MD, PhD, can authoritatively say that the 15th edition is the best. Released in March, this edition provides new information on ultrasound-guided peripheral intravenous cannulation, comprehensive instructions on proper hand hygiene, and a deeper dive into quality assurance.
Read More »Clinical Pathology Abstracts, 7/17
July 2017—Effects of early tranexamic acid administration on women with postpartum hemorrhage: The leading cause of maternal death is postpartum hemorrhage, which is defined as blood loss of more than 500 mL within 24 hours of giving birth. The majority of such deaths occur in low-income and middle-income countries.
Read More »Anatomic Pathology Abstracts, 7/17
July 2017—Potential quality indicators for lymph node staging of colon cancer: Evaluation of 12 or more lymph nodes is used as a quality indicator for adequacy of pathologic examination of colon cancer resections. The authors conducted a study to evaluate the utility of a focused lymph node search in the immediate vicinity of the tumor and a “second-look” protocol for improving lymph node staging in colon cancer.
Read More »Molecular Pathology Abstracts, 7/17
July 2017—Immune checkpoint inhibition therapy, such as blockage of PD-1/PD-L1 interaction, has proven effective in many types of cancers. The mechanism underlying this therapy is postulated to involve “disinhibition” of tumor-infiltrating lymphocytes that respond to neoantigens expressed by tumor cells. In theory, the larger the number of neoantigens expressed, the greater the immune response resulting from disinhibition.
Read More »Newsbytes, 7/17
July 2017—Machine learning: What will it do for pathology? If finance, online retail, and other industries are “embracing” machine learning, then the medical field is still in the polite handshake phase, despite the potential of this form of artificial intelligence to revolutionize health care. Recent research endeavors highlight just a few examples of what machine learning, which allows computers to analyze data, detect patterns, and build algorithms to guide decision-making, can contribute to the field of pathology alone.
Read More »Q&A column, 7/17
July 2017—A laboratory is considering the implementation of a laboratory test for the diagnosis of Zika virus infection. This test is currently labeled as a test under the issuance of an Emergency Use Authorization. What specific regulations regarding the use of this test, quality control, and proficiency testing apply when performing this test on patient specimens?
Read More »Put It on the Board, 7/17
July 2017—Putting pathology at the center of precision medicine: Michael H. Roehrl, MD, PhD, pathologist and director of the Precision Pathology Biobanking Center at Memorial Sloan Kettering Cancer Center, would like to see more joint development of companion diagnostics—pathologists and industry working together. It would lead, in his view, to better companion diagnostics.
Read More »From brain to umbilical cord tissue, next-gen mass spec
June 2017—Mass spectrometry has been gaining acceptance for routine assays in clinical laboratories. The 6th Annual AACC Conference on Mass Spectrometry and Separation Sciences for Laboratory Medicine, held in fall 2016, spotlighted best practices in the use of mass spectrometry testing for patient-centered care.
Read More »Diagnostics anchor freestanding ED
June 2017—To the business world, “appropriate technology” may evoke the era of tie-dyed shirts, bead curtains, and Mother Earth News. But the term, coined by Small Is Beautiful author E.F. Schumacher in 1973, comes close to describing the goal of health care systems as they opt to expand their facility footprint with freestanding emergency departments (FSEDs).
Read More »New scope for trial drives FDA verdict
June 2017—The new FDA-enabled milestone in pathology—approval in April of whole slide imaging for primary diagnosis—allows pathology to dip its toe into the technological revolution that has already transformed other fields. Widespread adoption will take time, training, and money, but it no longer awaits breakthrough approval.
Read More »Sleeping well in Seattle with lab’s shift to x-ray
June 2017—For laboratories and blood centers, thinking about safety is second nature, like rain in Seattle or sunshine in San Diego. Everyone understands the need to prevent transfusion-associated graft-versus-host disease, and standards, protocols, and habits have settled in to ensure a safe blood supply.
Read More »Family physician makes the case for CP consults
June 2017—He is a family medicine physician who did a residency and is board certified in clinical pathology. And even he asks his clinical pathology colleagues “day in and day out” which test to do and what to do with the results.
Read More »From the President’s Desk: Speaking of optics, 6/17
June 2017—We typically define “normal” or “true” from the perspective of our local communities and social circles. This reliance on the familiar can compromise communication effectiveness when we don’t appropriately consider the audience. And as the exchange of information continues to accelerate, the impact on what constitutes an authoritative assessment evolves similarly. Sometimes we don’t stop to think.
Read More »Kevin B. Dole, MD | 1948–2017
June 2017—Kevin B. Dole, MD, 68, a member of the CAP Board of Governors from 2001 to 2007, died April 23 of metastatic pancreatic cancer. He had served as a member of the Councils on Government and Professional Affairs and on Public Affairs and as chair of the Council on Membership and Professional Development. He was a member of more than a dozen committees, among them Credentials, CLIA Implementation, Practice Guidelines, and Federal and State Affairs (which he chaired). He was named Pathologist of the Year in 2009.
Read More »Book surveys patient safety from AP, CP standpoint
June 2017—CAP Press released in May Patient Safety in Anatomic & Clinical Pathology Laboratories. Editor Deborah Sesok-Pizzini, MD, MBA, and 11 additional contributors cover handoff communications, technology, tools and methods, human factors, a patient safety curriculum, and more. Dr. Pizzini is chief of blood bank and transfusion medicine in, and vice-chief of, the Department of Pathology and Laboratory Medicine, Children’s Hospital of Philadelphia. She is the department safety officer for CHOP Pathology and Laboratory Medicine, and she is a professor of clinical pathology and laboratory medicine, Perelman School of Medicine, University of Pennsylvania. We asked her about the new book. Here is what she told us.
Read More »Pathologist Cognition
June 2017—In the chapter “Diagnostic Errors and Cognitive Bias” in Patient Safety in Anatomic & Clinical Pathology Laboratories, Stephen S. Raab, MD, writes about pathology work process and cognitive failures, pattern recognition and cognitive strategies, interpretive error, and mitigation and improving safety. Here is his section on pathologist cognition. Dr. Raab is a professor of pathology at the University of Mississippi Medical Center, Jackson.
Read More »Helping phlebotomists ease pediatric patient anxiety
June 2017—“It’s the most talked about pain kids experience, even more so than post-op surgical pain.” Julie Piazza, a certified child life specialist, is referring to needlestick pain from pediatric blood draws. As project manager for patient and family-centered care at C.S. Mott Children’s Hospital in the University of Michigan health system, now known as Michigan Medicine, Ann Arbor, Piazza has observed anxiety at both ends of the needle.
Read More »Life-threatening bleeding—what’s the right call?
June 2017—In the CAP16 session, “Your Turn: Management of the Bleeding Patient,” Theresa Nester, MD, reminded attendees who provide transfusion medicine consultation to assess the available information before calling the clinical team: patient history, drugs, coagulation test results, and products administered so far. “Your main role is to help determine why the patient is bleeding and the most appropriate treatment,” said Dr. Nester, medical director of integrated transfusion services at Bloodworks Northwest in Seattle.
Read More »Emergency hemorrhage panel gives surgeons what they need
June 2017—As an alternative to point-of-care testing, Wayne Chandler, MD, and colleagues developed and implemented a rapid emergency hemorrhage panel, or EHP, for trauma patients (Chandler WL, et al. Transfusion. 2010;50[12]:2547–2552). The panel tests are prothrombin time, hematocrit, fibrinogen, and platelet count. “By limiting EHPs to patients that were actively bleeding, EHPs accounted for only 8 of 243 coagulation samples per day,” he and colleagues wrote in their 2010 article.
Read More »Anatomic Pathology Abstracts, 6/17
June 2017—Fallopian tube involvement in uterine serous carcinomas: The authors investigated the frequency and histopathologic and immunohistochemical characteristics of tubal involvement in uterine serous carcinoma to clarify the relationship between serous tubal intraepithelial carcinoma (STIC) and uterine serous carcinoma. They prospectively collected and reviewed, for the presence of tubal involvement, cases of the latter with complete tubal examination.
Read More »Clinical Pathology Abstracts, 6/17
June 2017—Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention: Cancers are caused by mutations that may be inherited or induced by environmental factors or that may result from DNA replication errors. The mutations due to random mistakes made during normal DNA replication may explain why cancers occur much more commonly in some tissues than others. Approximately three mutations occur every time a normal human stem cell divides.
Read More »Molecular Pathology Abstracts, 6/17
June 2017—Whole genome single-cell copy number profiling on FFPE tissue samples Single-cell genomic methods take the concept of analyzing intratumor genetic heterogeneity to its logical conclusion. Traditionally, however, single-cell methods can only be used to analyze fresh or rapidly frozen tissue because formalin fixation and paraffin embedding degrades tumor DNA and cross-links proteins.
Read More »Q&A column, 6/17
June 2017—Our analyzer reported nucleated red blood cells of six, with no cellular interference flag. The technologist missed that the automated NRBC was six. When he performed the manual differential, he noted more than five NRBCs and performed a corrected count and certified it. Is it acceptable to report out the automated white blood cell value as well as the corrected WBC?
Read More »Newsbytes, 6/17
June 2017—Making the most of classroom technologies to train residents: Google the phrase “millennials killed” and you’ll discover a genre of Internet clickbait claiming the generation in question has rejected a lengthy assortment of previously popular items, from “the suit” to “napkins” to the “hangout sitcom.”
Read More »Put It on the Board, 6/17
June 2016—CMS grants Qualified Clinical Data Registry status to Pathologists Quality Registry: The Centers for Medicare and Medicaid Services has approved the CAP’s Pathologists Quality Registry as a Qualified Clinical Data Registry, or QCDR. This makes it a reporting option for pathologists in fulfilling reporting requirements under Medicare’s Quality Payment Program.
Read More »Acute leukemia workups, from top to bottom
May 2017—Plenty can happen in five years. Just ask Cubs fans who watched their team leap from a 101-loss season in 2012 to a 103-win season in 2016 and a World Series title as the cherry on top. Or ask Daniel Arber, MD, who co-chaired a hefty new guideline—a half decade in the making—on diagnostic workup of acute leukemia. At the start of the project, “I think everyone going into it realized it was going to be a time-consuming, long process. But I don’t think anyone realized how long,” says Dr. Arber, professor and chair of pathology, University of Chicago, and the CAP co-chair for the guideline group.
Read More »TLA in, volume up—micro labs take stock
May 2017—Rise of the Robots. Disruption. Humans Need Not Apply. “The Future of Work.” A flood of books and articles in the past several months make the argument that service industries in the U.S. hover on the brink of total automation and humans will have to figure out how to adapt. Forty-five years ago, when Michael R. Jacobs, MD, PhD, started in microbiology, people fantasized about microbiology reaching this stage.
Read More »Whole slide imaging for primary diagnosis: ‘Now it is happening’
May 2017—When the Food and Drug Administration granted permission to Philips to market its whole slide imaging system for primary diagnosis last month, it was a “big deal” of the highest order. “Yes, this is a very big deal,” says Liron Pantanowitz, MD, a professor of pathology and biomedical informatics at the University of Pittsburgh Medical Center. “This event will provide the impetus to drive digital pathology forward for clinical use in the U.S., and allow us to catch up with our colleagues around the world who are ahead of us in their digital transformation journey.”
Read More »Study ‘opens the door’ to troponin, diabetes link
May 2017—Clinicians and laboratories have only begun to wade into the depths of the FDA’s long-awaited clearance of a new-generation, high-sensitivity cardiac troponin T (hs-cTnT) assay for rapid diagnosis of acute myocardial infarction. Roche’s Elecsys TnT Gen 5 STAT assay received just such clearance in January. Yet researchers are already deep into investigations that may float new opportunities for high-sensitivity troponin T testing to the surface of medical diagnostics.
Read More »From the President’s Desk: Soup’s ready, ketchup plopped, 5/17
May 2017—When we were growing up, my sisters would often ask, “Is it soup yet?” mimicking a popular TV commercial. The catch phrase caught on with the kids as a way to express eagerness for anything, lunch related or not. Another commercial popular back then showed an inverted ketchup bottle slowly yielding its contents to the force of gravity while Carly Simon belted out “Anticipation.” Part of me always wanted to tell my friends that because ketchup was thixotropic, a good shake would help the ketchup flow faster. I never did, probably because that wasn’t how the cool kids talked and partly because it seemed necessary to wait for the ketchup to plop.
Read More »In memoriam: Richard E. Horowitz, MD | 1931–2017
May 2017—Richard E. Horowitz, MD, a member of the CAP Board of Governors from 1997 to 2000, died March 15 at age 85 of complications from lung cancer. Dr. Horowitz is a past member of the CAP’s House of Delegates and the CAP’s Practice and Education, Government Affairs, and Public Affairs councils. He was chair of the Outcomes and Performance Measures committees and a longtime member of the Committee on Computerized Laboratory Systems. He was a member, vice president, and president of the CAP Foundation Board of Directors.
Read More »In flu season management, POC molecular to the fore
May 2017—Stacked against some of the nation’s previous bouts with influenza—such as the 2014–15 season—the 2016–17 flu season didn’t break records for drama. To be sure, every flu season is different, and regional variation was prominent. In Central Texas, some outbreaks appeared to start later than usual, but the dominant viruses were the same as last year’s—H1N1, H3N2, and influenza B—says Bob Fader, PhD, chief of the virology and microbiology laboratory at Baylor Scott & White Health, Temple, Tex. The strains identified were a good match with this year’s trivalent and quadrivalent vaccine. Testing volume was up, as were positive PCRs.
Read More »How billing systems profit from analytics and automation
May 2017—The laboratory financial systems of yesteryear were built to deliver on a prime directive: achieve optimal, timely payment. Fast-forward to today and the overriding goal remains largely the same, but the means to the end has become more sophisticated, with billing/accounts receivable/revenue cycle management systems providing capabilities to recover outstanding payments, pinpoint reimbursement bottlenecks, and deliver a diverse range of data.
Read More »For C. difficile, lab assessment alone is not enough
May 2017—Toxigenic Clostridium difficile can be isolated in about one-third of hospital rooms in which there is no patient with C. diff infection, and the same is seen in the community. A study published in 2014 found that 32 percent of the samples obtained from 30 houses in Houston were culture-positive for toxigenic C. diff. And C. diff was isolated from 83 percent of the houses.
Read More »No perfect approach to detecting C. diff infection
May 2017—With Clostridium difficile causing a wide range of infectious manifestations, the dilemma for clinical laboratories is how to balance the different diagnostic options. “Because if you’re treating someone who is only colonized, you’re not going to benefit them—and very likely you may harm them,” said Ferric C. Fang, MD, professor of laboratory medicine, Department of Microbiology and Medicine, University of Washington School of Medicine, Seattle, in a recent webinar hosted by CAP TODAY and sponsored by BioFire. And having a negative toxin assay is no assurance, he said, that C. difficile is not causing disease.
Read More »Cytopathology in Focus: More aggressive follow-up for patients with AGC?
May 2017—The most recent edition of the Bethesda System for Reporting Cervical Cytology classifies glandular cell abnormalities into the following broad categories: atypical (specify favored site of origin), atypical (favor neoplastic), endocervical adenocarcinoma in situ (AIS), and adenocarcinoma.1 Generic terminology of “atypical glandular cells (AGC)” may be used if the origin of the cells cannot be determined with certainty. Nevertheless, the Bethesda System encourages pathologists and cytotechnologists to report the favored site of origin (endometrial versus endocervical) whenever possible.
Read More »Cytopathology in Focus: Integrating cytology samples into molecular testing of tumors
May 2017—Use of cytology specimens for molecular testing can spare patients from repeated or more invasive procedures. A two-part special section in recent issues of Archives of Pathology & Laboratory Medicine highlights a variety of applications of molecular techniques in cytopathology specimens. The articles in this section cover laboratory workflow issues, considerations for the preparation of cell blocks, application of immunoperoxidase staining and FISH to cytology specimens, and specific applications of molecular testing in thyroid and lung specimens.
Read More »Cytopathology in Focus: Paris System for urinary cytology: why and where now
May 2017—It is well known that examination of urine dates back to antiquity, but it wasn’t until the 19th century that cancer cells were microscopically documented in urine, by Hermann Lebert in 1845 and Vilem D. Lambl in 1856. Over many decades, countless talented and noteworthy authors have contributed valuable observations and conceptual mechanisms to the study of urinary cytology, but a systematic, universally accepted, internationally recognized system with clear goals was missing.
Read More »New autopsy book ‘a complete learning experience’
May 2017—Autopsy Performance & Reporting is a new book from CAP Press, released in April. The editor, Kim A. Collins, MD, and her 43 contributors wrote 40 chapters on facility design, safety, high-risk cases, the oral cavity, the placenta, the pediatric autopsy, special studies of the heart and lungs, postmortem microbiologic testing, photomicrography, and much more. “I know of no other autopsy book like this on the market,” Dr. Collins tells CAP TODAY.
Read More »Pregnancy-related death: Hepatic System
The chapter in Autopsy Performance & Reporting titled “Pregnancy-Related Death and the Autopsy Examination” is written by Cynthia Schandl, MD, PhD, of Medical University of South Carolina. Here from that chapter is an excerpt (published without references) on the hepatic system. Dr. Schandl’s chapter also covers the cardiovascular, respiratory, hematopoietic, urogenital, and endocrine systems, as well as the gastrointestinal tract and skin and connective tissue.
Read More »Clinical Pathology Abstracts, 5/17
May 2017—Viral hepatitis and Parkinson disease; Case studies of professionalism in pathology as an educational tool
Read More »Anatomic Pathology Abstracts, 5/17
May 2017—Value of Ki-67 proliferative index in WHO-classified pulmonary carcinoids; Chromosomal abnormalities and genetic changes in uterine smooth muscle tumors; Expression of divergent endodermal lineage markers in yolk sac tumors; Interobserver reproducibility of percent GP4 in prostatic adenocarcinoma on biopsies; MELF pattern invasion: a report of FIGO grade 1 endometrial endometrioid adenocarcinomas; Cost-effectiveness of identifying H. pylori in gastric biopsies without ancillary stains
Read More »Molecular Pathology Abstracts, 5/17
May 2017—Contribution of tumor microenvironment to cancer phenotype after DNA damage; BRCA1 and metabolism: Partners in the development of ovarian cancer?
Read More »Q&A column, 5/17
May 2017—Is there any medical reason why a physician would ask the lab to run a complete blood count on cord blood? Does CAP checklist requirement HEM.23050 treat automated and manual differentials equally? That is, does the recommendation to report absolute counts apply also to manual differentials or only to automated differentials? What is the next step in resolving platelet clumping when it occurs in a citrate tube also?
Read More »Newsbytes, 5/17
May 2017—What R and Python programming languages bring to the table; Philips and PathAI partner on artificial intelligence offerings; Hc1.com and 4medica announce collaboration; Technidata releases new generation of middleware
Read More »Put It on the Board, 5/17
May 2017—First here, then there—FISH testing in Kenya; FDA OKs PD-L1 biomarker test for urothelial carcinoma; FDA clears Roche Cobas e 801 immunoassay module; DiaSorin granted EUA for fully automated Zika IgM test; Advia Centaur XPT has comprehensive ID menu; Roche CINtec Histology test receives FDA clearance
Read More »New molecular road map for CRC
April 2017—Molecular testing for colorectal cancer is not for the faint of heart. While that’s not news to Stan Hamilton, MD—he’s head, Division of Pathology and Laboratory Medicine, and the Frederick F. Becker distinguished chair in cancer research, University of Texas MD Anderson Cancer Center—he was reminded of this fact recently when a friend looked at the multipage molecular pathology report on his own tumor.
Read More »With cloud computing, sorting out pros, cons
April 2017—“No man putteth new wine into old wineskins” reads the biblical aphorism in Luke 5:36–39, which continues by giving the reason: “The new wine would burst the skins and be spilled, and the skins would perish.” Old wineskins, biblical scholars say, would typically be stretched to the limit or become brittle as wine had fermented in them.
Read More »Primary aldosteronism: diagnostic team lifts clinical practice
April 2017—For decades, Michael Laposata, MD, PhD, chair of pathology at the University of Texas Medical Branch in Galveston, has touted the value of diagnostic management teams, and in February he led the first conference dedicated to such teams, referred to as DMTs. There, Alison Woodworth, PhD, told the story of how and why she created a DMT for primary hyperaldosteronism, what it achieved, and where her DMT focus is now.
Read More »From the President’s Desk: If you can’t find your niche, build it, 4/17
April 2017—Our ubiquitous access to (and increasing reliance on) search engines such as Google, Siri, Cortana, and others has resurfaced much of the intellectual landscape. The historical “Guardians of the Truth” no longer sit comfortably in ivory towers waxing poetic while issuing definitive determinations of “fact.” Today, when we want to know something, we reach for our phones. The answers, devoid of context and authoritative citations, seem to be so simple there.
Read More »Hemophilia management: Tips on monitoring modified replacement therapies
April 2017—Some modified recombinant factor VIII and IX products for hemophilia prophylaxis show significant reagent-dependent recovery in the one-stage assay, while recovery in the chromogenic assay appears to be more consistent, especially for modified recombinant factor IX. The variable results can lead to over- or underestimating the factor level, warn Stefan Tiefenbacher, PhD, of Colorado Coagulation, and Rajiv K. Pruthi, MBBS, of Mayo Clinic.
Read More »Managing population health takes on a new look
April 2017—Quarantine, antisepsis, sanitation, vaccination. Over more than a century and a half, as these staples of public health have evolved, they have proved that stunning improvements in general health status can result from adopting broad public policies based on data and statistical analysis.
Read More »Drug-induced injury: liver pathology’s big imitator
April 2017—In a presentation at CAP16 on common patterns of liver injury, Robert M. Najarian, MD, called drug-induced liver injury the great mimicker in liver pathology. “When in doubt, rule drugs out,” he said, including herbal agents, supplements, and over-the-counter agents.
Read More »Assessing LPL software
April 2017—Twenty years ago, CAP TODAY released its first product guide for laboratory-provider links software. The demand for connectivity was growing as laboratories built their outreach business, and the future looked bright for LPL software companies.
Read More »Setting the record straight on Maintenance of Certification
April 2017—The American Board of Pathology has been committed to implementing a Maintenance of Certification program for its diplomates that addresses all of the program’s components in a manner that is the least burdensome and most relevant to participating diplomates. This is an iterative process that incorporates feedback from participating diplomates.
Read More »Clinical Pathology Abstracts, 4/17
April 2017—Cost-benefit analysis of Chlamydia trachomatis screening in pregnant women: Chlamydia trachomatis is the most common bacterial sexually transmitted infection in the United States. In 2010, more than 1.3 million such infections in the United States were reported to the Centers for Disease Control and Prevention. In 2013, the estimated direct lifetime cost of treatment for chlamydia and its complications was more than $500 million.
Read More »Anatomic Pathology Abstracts, 4/17
April 2017—Proposing prognostic thresholds for lymph node yield in oral cavity cancers: Prognostic lymph node yield thresholds have been identified and incorporated into treatment guidelines for multiple cancer sites, but not for oral cancer. The authors conducted a study to identify optimal thresholds in elective and therapeutic neck dissection for oral cavity cancers.
Read More »Molecular Pathology Abstracts, 4/17
April 2017—Molecular profiling in MDS to predict clinical outcomes after transplantation: In recent years, several insights have been gleaned regarding the role of molecular markers for prognosis in myeloproliferative disease. This study expanded the use of molecular markers for prognosis in myelodysplastic syndrome (MDS) to predicting clinical outcomes after allogeneic hematopoietic stem-cell transplants.
Read More »Q&A column, 4/17
April 2017—Our laboratory receives requests for breast predictive marker testing (estrogen receptor, progesterone receptor, HER2, Ki-67) on biopsies of bone metastases. Is it appropriate to perform this testing on decalcified tissue? Is there a regulatory speed limit—whether a per day or a per hour “at the microscope” workload limit—on surgical pathology slide interpretations, similar to workload limits for cytology screening?
Read More »Newsbytes, 4/17
April 2017—New open-access website offers a treasure trove of digital slides; HL7 collaborates with Google; Health Catalyst and Regenstrief to advance text analytics technology; Corista teams with Elsevier to augment digital platform; Seacoast enhances system for document management
Read More »Put It on the Board, 4/17
April 2017—At OSU, Inspirata completes deployment of WSI scanners, launches Consultation Portal: Inspirata has completed what it describes as the largest single-site deployment of whole slide image scanners at the Arthur G. James Cancer Hospital and Richard J. Solove Research Institute (The James) and the Department of Pathology, both located at Ohio State University Wexner Medical Center, Columbus.
Read More »New rays on blood safety
March 2017—The language of blood banking experts, as they talk about irradiators, transfers easily to a car dealership. How reliable are the newer models? Are you willing to replace it every 10 years or so? Do you keep running it until it dies? What parts are likely to burn out? What will repairs run? And then the word “terrorism” pops up.
Read More »Lower HbA1c seen with sickle trait, but questions remain
March 2017—Perhaps unusually for news about clinical diagnostics research, an article in the Feb. 7 issue of JAMA created a mild stir with findings that HbA1c results in patients with sickle cell trait, the most common hemoglobin variant in the U.S., may systematically underestimate past glycemia (Lacy ME, et al. 317[5]:507–515).
Read More »Cell-free DNA screening blooms in expansion to low-risk pregnancies
March 2017—Something about having the letters “DNA” in a test’s name may make the test seem like the last word, the key to a black-and-white, definitive diagnosis. That connotation has been problematic for cell-free DNA sequencing used for noninvasive prenatal testing, because the test is not intended or designed for diagnosis, but only for screening. It’s for that reason, in fact, that some maternal-fetal medicine specialists and clinical geneticists prefer to use the term “noninvasive prenatal screening,” with the acronym NIPS.
Read More »Hemophilia diagnosis: how to test, what to know
March 2017—True, hemophilia is no longer commonly known as the “royal disease” (as it was when several generations of European rulers suffered from it). But in a January webinar, Dorothy M. Adcock, MD, gave some royally important suggestions regarding the laboratory diagnosis of hemophilia A and B.
Read More »From the President’s Desk: We all need a safe place, 3/17
March 2017—Students of history tell us that a durable paradigm shift most often involves a long gestation, typically under the radar and recognized by a precious few. What they frequently fail to tell us is that the vast majority of prognosticators often turn out to be both flat-out wrong and invisible when the “future” arrives. Either way, however, the prevailing narrative points to uncertainty in the greater health care environment.
Read More »HbA1c in CVD treatment: farewell to one size fits all
March 2017—Anchor. Central pillar. Cornerstone. It would be hard to find a weighty synonym for “linchpin” that hasn’t been used to describe HbA1c’s role in diabetes diagnosis and management since 2010, when the assay was recognized by key standard-setting organizations as the equal of fasting glucose and oral glucose tolerance testing in diabetes and prediabetes testing.
Read More »Liver injury patterns: pitfalls and pointers
March 2017—Keep eyes wide open to everything and describe everything because there might be a number of different factors playing into a patient’s disease. That was the reminder Robert M. Najarian, MD, opened with last fall in his CAP16 presentation on common patterns of liver injury.
Read More »Dashboard eases performance analysis and prep
March 2017—Crystal Sands, MBA, MT(ASCP)SM, manager of quality, regulatory, and safety at NorDx Laboratories in Scarborough, Me., has a new favorite product, and she’s not shy about saying so. “Oh my gosh, I’ll be playing with it for a long time,” she says. “Every time I use it, I find different ways to slice and dice.”
Read More »Clinical Pathology Abstracts, 3/17
February 2017—Preventing genetic testing order errors via a lab utilization management program: Diagnostic errors, or failure to provide an accurate and timely diagnosis, impact an estimated 12 million outpatient care visits annually in the United States. These errors can often be attributed to the testing process, including test selection, ordering, retrieval, and interpretation. Literature about diagnostic errors has primarily focused on the outpatient setting; study of diagnostic error in the inpatient setting has been limited.
Read More »Anatomic Pathology Abstracts, 3/17
March 2017—An immunohistochemical algorithm for ovarian carcinoma typing: Five major histotypes of ovarian carcinoma exist. Diagnostic typing criteria have evolved over time, and past cohorts may be misclassified by current standards. The authors undertook an endeavor to reclassify the recently assembled Canadian Ovarian Experimental Unified Resource and Alberta Ovarian Tumor Type cohorts using immunohistochemical (IHC) biomarkers and to develop an IHC algorithm for ovarian carcinoma histotyping.
Read More »Molecular Pathology Abstracts, 3/17
March 2017—Effects of ovarian cancer cells manipulating mesothelial cells that line the peritoneal cavity: spread within the peritoneal cavity, resulting in cell implantation and metastasis at many secondary sites. The peritoneal cavity and associated organs are lined by a single layer of mesothelial cells that it has been suggested not only provides a physical barrier to prevent implantation and invasion but also plays a more complex interactive role in regulating cancer spread.
Read More »Newsbytes, 3/17
March 2017—Pathologist to ‘name names’ in support of interoperability: Desperate times call for desperate measures. And while desperation may be a bit of an overstatement, a leader in pathology informatics says extreme frustration with LIS vendors, whose closed architecture, in effect, holds client data hostage, drove him to rally his colleagues to call out the offenders in a public forum.
Read More »Q&A column, 3/17
March 2017—Our hospital system is implementing Sysmex instruments with a focus on the accuracy of the absolute white blood cell values—use of the absolute neutrophil count and immature granulocytes with the WBC as markers for septicemia. I then became aware that the hospital purchased the St. John Sepsis v14 protocol, which lists 10 percent bands as one of the markers for septicemia. The Rumke for 10 percent is 4–16. Using bands is not consistent with reducing manual differentials and is not an accurate parameter to use. Are there other protocols using WBC/ANC?
Read More »Put It on the Board, 3/17
March 2017—LabCorp to acquire PAML: LabCorp, Providence Health & Services, and Catholic Health Initiatives announced Feb. 23 they have entered into a definitive agreement for LabCorp to acquire all of the ownership interest in Pathology Associates Medical Laboratories, LLC, which is owned by Providence and CHI.
Read More »Latest TB testing guide set forth by ATS, CDC, IDSA
March 2017—Testing for latent Mycobacterium tuberculosis infection and active tuberculosis disease remained relatively unchanged for many years. Screening for latent infection depended on an initial positive tuberculin skin test, and evidence for active TB required a positive culture for M. tuberculosis complex. New tests altered this picture in the past five years. For diagnosis of latent infection, interferon-gamma release assays have taken a major role. And nucleic acid amplification testing is becoming a mainstay for establishing a diagnosis of TB.
Read More »AMP case report: February 2017 test yourself answers
March 2017—In the February 2017 issue was a report, “An unusual BRAF mutation in a patient with melanoma,” written by members of the Association for Molecular Pathology. Here are answers (in bold) to the three “test yourself ” questions that followed that case report.
Read More »In cancer sequencing, a new lingua franca
February 2017—NGS has taken its NBS, or next big step: a newly published joint consensus guideline on how to interpret and report sequence variants in cancer. With these 20 pages of best practices for making next-generation sequencing a regular part of cancer diagnostics, the field is moving, essentially, from frontier town to gated community.
Read More »Guidelines reset horizons of molecular testing in NSCLC
February 2017—It doesn’t happen often. But from time to time, says Gene Finley, MD, director of medical oncology at Allegheny Health Network in western Pennsylvania, a patient who is at death’s door will make such a dramatic recovery with therapy that clinicians refer to it as a “Lazarus effect.”
Read More »One bug or prix fixe? Respiratory pathogen testers weigh in
February 2017—With the number of rapid, accurate molecular assays for respiratory pathogens growing, microbiology laboratories have more options than ever. They include, among others, Meridian Bioscience’s Illumigene assays for group A Streptococcus and pertussis and its newest assay, Mycoplasma Direct, as well as Alere’s assays for influenza A/B, respiratory syncytial virus, and Streptococcus on its i molecular platform. No longer are laboratories limited to inaccurate rapid antigen tests, weeks-long culture, or multi-pathogen panels.
Read More »OIG warns against selective free labeling services
February 2017—A laboratory’s proposal to provide free labeling services to some of its dialysis center clients poses more than a minimal risk of fraud and abuse, according to an advisory opinion from the Office of Inspector General of the U.S. Department of Health and Human Services.
Read More »From the President’s Desk: The deep roots of tall trees, 2/17
February 2017—One of my mentors, Richard E. Horowitz, MD, is thoughtful, perceptive, persuasive, and calmly persistent. He is a pathologist to the core, the physician inseparable from the scientist. When he wants to know something, he asks—and there’s plenty he wants to know. He is an emeritus professor of pathology with a long string of leadership credits, so he knows where to look for answers to deep questions. And his seemingly irrepressible inclination to mentor younger pathologists nurtures pragmatic leaders who can move our specialty forward.
Read More »AMP v Myriad: driving or disrupting innovation?
February 2017—The Association for Molecular Pathology belongs to a small and exclusive club of plaintiffs on the winning side of a unanimous U.S. Supreme Court decision. Such a ruling was issued in 2013 in the case of AMP v Myriad Genetics, a suit sponsored by the American Civil Liberties Union with the AMP as lead plaintiff.
Read More »In AP systems marketplace, software comes and grows
February 2017—Software for anatomic pathology has evolved mightily since 1987, the year that CAP TODAY set sail on its maiden voyage as a monthly publication. During that year, HL7 was founded and the Co-Path system, then the flagship product of Collaborative Medical Systems, made a terrific splash on the small exhibit floor at the Palmer House Hotel in Chicago.
Read More »AMP case report: An unusual BRAF mutation in a patient with melanoma, February 2017
February 2017—An activating BRAF mutation is found in 40 to 60 percent of melanoma patients. BRAF (B-Raf proto-oncogene) encodes a protein-kinase that activates the MAP kinase/ERK signaling pathway, a pathway that regulates cell differentiation, growth, and survival. Another protein, NRAS, normally activates BRAF. A mutated BRAF, however, can act independently of NRAS and skew cell activity toward growth and survival and away from differentiation.
Read More »‘A marriage of virtual and real bronchoscopy’
February 2017—The molecular testing guidelines have been having a significant impact on surgical practices since they were issued, said thoracic surgeon Min Kim, MD, another webinar panelist. As practice at his institution, Houston Methodist Hospital, has evolved, Dr. Kim said, there has been an increasing need for minimally invasive ways of obtaining tissue from lung cancer patients.
Read More »Book review: ‘Resource of choice’ for quality management in AP
February 2017—“Quality management” is the practice of continually evaluating, identifying, and improving the diagnostic process. It refers not only to retrospective action taken after mistakes have been made but also to evaluating near misses and opportunities for improvement in every facet of practice.
Read More »Molecular Pathology Selected Abstracts, 2/17
February 2017—Concordance between liquid biopsy and patient-matched tumor molecular testing: The use of sequence analyses of extended panels of genes to identify therapeutic targets in cancer is becoming commonplace. These assays typically rely on the availability of tissue biopsies as a source of genomic material, which can become a limitation in situations where insufficient tissue is available or an invasive procedure to collect tissue is impractical. A potential solution to this dilemma is the use of a blood-based, or liquid biopsy, approach, in which a peripheral blood sample is used as a source of tumor-derived genomic material, either in the form of cell-free DNA (cfDNA) or via circulating tumor cells (ctcDNA).
Read More »Anatomic Pathology Abstracts, 2/17
February 2017—Findings in hysterectomy specimens of women with Lynch syndrome; Percentages and architectural types of Gleason pattern 4 cancer in radical prostatectomy; PTEN loss and chromosome 8 alterations in Gleason grade 3 prostate cancer cores; Lymph node count from neck dissection predicts mortality in head and neck cancer; Assessing the adequacy of lymph node yield for papillary thyroid cancer; Addressing perceived versus actual agreement in breast pathology interpretation; Cost-effectiveness of Oncotype DX DCIS score for guiding treatment of DCIS
Read More »Clinical Pathology Abstracts, 2/17
February 2017—Screening for Babesia microti in the U.S. blood supply; Perspectives on whether WES is ethically disruptive in pediatrics; Perspectives on whether WES is ethically disruptive in pediatrics
Read More »Q&A column, 2/17
February 2017—I have an oncology patient with a diagnosis of immune thrombocytopenia. The patient’s sample has been drawn in sodium citrate, EDTA K2, sodium heparin, and warm saline replacements, and a true platelet count cannot be obtained. Platelets clump in all tubes, and multiple platelet clumps are observed under the microscope. The patient doesn’t have thrombocytopenia. What else can I do?
Read More »Newsbytes, 2/17
February 2017—In virtual informatics conference series, students teach and learn: In the television drama “The Paper Chase,” law professor Charles W. Kingsfield strikes fear and terror in the hearts of his students by “cold-calling” on them in class. Douglas Bell, MD, PhD, professor of medicine at the University of California, Los Angeles, and the program director of UCLA’s clinical informatics fellowship program, doesn’t want his own classes to be as stressful. But part of the pedagogical challenge for Dr. Bell and Bruce Levy, MD, who together run a virtual conference series for clinical informatics fellows, is finding a way to use active learning techniques, like calling on students, when the students are remote.
Read More »Put It on the Board, 2/17
February 2017—LabCorp to purchase Mount Sinai’s outreach laboratories; Philips and Illumina to offer integrated genomics solutions for oncology; Werfen, IL acquire Accriva; Abbott granted EUA for molecular Zika test; Guideline now out on CRC molecular biomarker testing
Read More »Cracking the many mysteries of HER2 GEA
January 2017—Only a sadist would want to see gastroesophageal adenocarcinoma become as common as breast cancer. GEA wreaks enough destruction already as the fifth (stomach) and eighth (esophageal) most common cancers worldwide.
Read More »In new era, cannabis testing a mixed bag
January 2017—Extended cruises down the rivers of Europe and life without alarm clocks might figure in a vision of retirement for some people, but don’t include toxicology expert Marilyn A. Huestis, PhD, in that contingent, at least for now.
Read More »Buzz, prospects build for heparin-induced thrombocytopenia test
January 2017—U.S. physicians and laboratories are anticipating the early 2017 launch of the HemosIL HIT-Ab(PF4-H) assay, which detects antibodies associated with heparin-induced thrombocytopenia. The new test from Instrumentation Laboratory, Bedford, Mass., is the first fully automated, on-demand assay for HIT.
Read More »‘Brave’ new book—AP quality management for everyone
January 2017—What does it take to produce and edit the first book on AP quality management that the CAP has published in more than a decade? A diverse network of experts, a commitment to comprehensive quality assurance, and, says co-editor Qihui “Jim” Zhai, MD, a bit of bravery.
Read More »From the President’s Desk: The constancy of uncertainty, 1/17
January 2017—In February 2002, with Americans still reeling from the Sept. 11 attacks, many were preoccupied with rumors suggesting that the Iraqi dictator had acquired weapons of mass destruction and intended to supply them to terrorists.
Read More »With metagenomic sequencing, no pathogen can hide
January 2017—Detecting pathogenic organisms with PCR has become a staple of the clinical microbiology laboratory, so much so that it seems like it has always been there. A more advanced molecular technique—unbiased metagenomic next-generation sequencing—will increasingly become a part of infectious disease diagnosis because it has several advantages over PCR. While it will be demanding to perform at first, it, too, may become a standard method in the clinical microbiology laboratory.
Read More »Cytopathology In Focus: The significance of NIFTP for thyroid cytology
January 2017—A recent landmark study performed under the auspices of the Endocrine Pathology Society has proposed a new diagnostic entity in the thyroid: noninvasive follicular thyroid neoplasm with papillary-like nuclear features, or NIFTP.
Read More »Cytopathology In Focus: NIFTP’s impact on FNA malignancy risk
January 2017— What’s in a name? As announced in JAMA Oncology in April 2016, tumors previously classified as follicular variant of papillary thyroid carcinoma and found to have no invasion on adequate sampling of the tumor capsule have been given a new name: noninvasive follicular thyroid neoplasm with papillary-like nuclear features, or NIFTP.
Read More »Cytopathology In Focus: CAP meets MOC with à la carte modules online
January 2017—It’s the end of the year, say, and you are just a few self-assessment modules, or SAMs, short of the required 20 in your area of expertise—anatomic pathology. You have to meet the requirements for the American Board of Pathology’s Maintenance of Certification and time-limited cytopathology and AP/CP certificates for each two-year reporting cycle. What to do?
Read More »Cytopathology in Focus: The dysfunctional relationship between labs and their IT
January 2017—Complaints about laboratory information technology services are nearly universal. Rare is the person who is happy with his or her laboratory information system or with the way information services are delivered.
Read More »Tough times demand superior business savvy
January 2017—Reimbursement policies, competitive forces, and pathologist workforce numbers will reshape pathology groups in the coming years, say Barry Portugal, president of Florida-based Health Care Development Services, and Edward P. Fody, MD, president of Western Michigan Pathology Associates.
Read More »The what and why of diagnostic management teams
January 2017—Michael Laposata, MD, PhD, has been speaking for years about the need for laboratory consultations and diagnostic management teams, and he will lead the first formal meeting Feb. 7–8 in Galveston, Tex., on what the teams are and how to implement them. Writer Ron Shinkman put a few questions to him about diagnostic management teams and pathology practice. Dr. Laposata is a professor in and chairman of the Department of Pathology, University of Texas Medical Branch-Galveston. Here’s what he said.
Read More »Molecular Pathology Selected Abstracts, 1/17
January 2017—Tumor profiling and patient outcomes in genotype-matched clinical trials: Molecular profiling of tumors with next-generation sequencing can provide important diagnostic and prognostic information that can be used to inform treatment strategies.
Read More »Anatomic Pathology Abstracts, 1/17
January 2017—Drawbacks of reflex ER and PR analysis of DCIS in breast needle core biopsies; Analysis of eosinophils and mast cells of gastrointestinal tract in healthy children; Classifying gastric cancer into molecular subgroups; Clinicopathologic significance of mismatch repair defects in endometrial cancer; Use of immunostains to distinguish hepatic adenoma from hepatocellular carcinoma; Prognostic effect of PD-L1 expression patterns in cervical cancers; Variation in pattern-based classification of invasive endocervical adenocarcinoma
Read More »Clinical Pathology Abstracts, 1/17
January 2017—Impact of laboratory cost display on resident attitudes and knowledge of costs: The Institute of Medicine report on health care quality recommends providing better care at lower costs. However, the United States has consistently seen rising health care costs instead of cost reductions. An approach to reducing unnecessary health care spending is to make physicians more aware of the cost of diagnostic tests.
Read More »Q&A column, 1/17
January 2017—I have a technologist who is a recent graduate from a medical technology school. She has her BA but the school she attended did not offer an internship program. We are offering her one year of on-the-job training so she will be able to sit for her ASCP certification exam after completing the one year of training.
Read More »Newsbytes, 1/17
January 2017—How informatics tools can boost QA in anatomic pathology: Love and marriage, cookies and milk . . . quality assurance and informatics? In pathology, the latter pair are a natural fit, says Liron Pantanowitz, MD.
Read More »Put It on the Board, 1/17
January 2017—Experts collaborate on evidence-based somatic variant classification system; FDA clears next-generation test for MRSA colonization; PMA approval granted for Aptima HIV-1 Quant; Henry Ford announces automation partnership; ArcherDX launches immune repertoire sequencing assays
Read More »AMP case report: December 2016 test yourself answers
In the December 2016 issue was a report, “Isolated hepatic neuroendocrine tumor expressing albumin mRNA and arginase-1,” written by members of the Association for Molecular Pathology. Here are answers (in bold) to the three “test yourself ” questions that followed that case report.
Read More »Procalcitonin passes automation hurdle
December 2016—Matt Damon in Interstellar. Julia Roberts in The Player. Gene Hackman in Young Frankenstein. When movie stars appear in uncredited parts, it’s usually for a cameo, not a leading role. But in diagnostics, an uncredited or off-label use of an assay might be its main use—possibly even its most clinically important use.
Read More »Highs, lows of immune checkpoint inhibitors
December 2016—Barry Nelson was the first in his cancer patient support group to undergo immunotherapy, which at the time was in a phase one clinical trial at Dana-Farber Cancer Institute. As the therapy became available to others in his group, they would ask for his advice on whether to try it. For the answer, he’d suggest they consult with their doctors and pray. He’d add, “I hope if you decide to go with it that you’ll have the same results that I do.”
Read More »Lymphoid neoplasms: Steven Swerdlow on classification revisions
December 2016—In his CAP16 talk, “Lymphoma Diagnosis and Classification: My Search for the Holy Grail,” Steven H. Swerdlow, MD, acknowledged that the quest has been long and contentious and the resulting classification complex. Why, he asked, is lymphoma classification so complex? It reflects an explosion of knowledge about the immune system and lymphomas and an increasing number of therapeutic targets requiring increasingly precise diagnoses. In other words, it reflects the complexity of the disease itself. As oncologist Alan C. Aisenberg, MD, PhD, wrote in 1995, “The complexity of non-Hodgkin’s lymphoma reflects the complexity of the lymphoid system.”
Read More »Is the value of hospital lab outreach underrated?
December 2016—Hospitals across the nation have been selling their laboratory outreach operations to national laboratories, which have been snapping them up from community hospitals and larger enterprises. Take Henry Mayo New-hall Hospital in California (LabCorp) and Hartford HealthCare in Connecticut (Quest Diagnostics) this year, for example, and MemorialCare in California (Quest) in 2015.
Read More »Medicare revises 2017 discount on add-on codes: Increases professional, cuts technical, pay for prostate biopsies
December 2016—Overall Medicare reimbursement for some professional pathology services will rise in 2017 due to increases sought by the CAP for add-on codes and other services provided by pathologists to Medicare beneficiaries. At the same time, the Medicare program will move forward with cuts affecting the technical component of pathology services, including flow cytometry, due to a targeted revaluation mandated by federal law.
Read More »New protocols on deck as pathology helps reshape cancer staging
December 2016—It’s a familiar campus lament. New editions of textbooks in some academic fields have become notorious for providing little more than a few extra paragraphs of text or a few more references and altered pagination—mainly, students suspect, to serve as a damper on the textbook resale market. What is happening with a key text in the field of cancer care, however, is in marked contrast. The changes contained in the 8th edition of the AJCC Cancer Staging Manual of the American Joint Committee on Cancer, slated to take effect Jan. 1, 2018, are the opposite of cosmetic.
Read More »AMP case report: Isolated hepatic neuroendocrine tumor expressing albumin mRNA and arginase-1, December 2016
December 2016—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from the University of Pittsburgh Medical Center.
Read More »What molecular diagnostics laboratory systems offer
December 2016—As personalized and predictive medicine have progressed from not-in-my-lifetime to now available, health care information technology vendors have faced the challenge of how to manage a mass of molecular data and direct molecular testing processes. CAP TODAY asked vendors of molecular diagnostics lab information systems to explain what their products contribute to the flourishing field of molecular testing. Here are their responses.
Read More »Recent approvals and the pipeline
December 2016—Pembrolizumab (Keytruda) is now FDA approved as monotherapy for patients with untreated metastatic NSCLC, both squamous and nonsquamous histologies, that express PD-L1 at a high level of 50 percent or more, says Dr. Roger Dansey, who headed up Keytruda development at Merck Research Laboratories. “We do not have data at this point that would say whether a cutpoint lower than 50 percent in the first-line setting would be of equal value in terms of response to Keytruda,” he says.
Read More »Molecular Pathology Selected Abstracts, 12/16
December 2016—ANXA1 as a predictive biomarker for resistance to trastuzumab in breast cancer: Treatment with the HER2-targeting antibody trastuzumab (Herceptin) is a key component of therapy for women with HER2-positive breast cancer. However, a subset of women with advanced disease shows initial or acquired resistance to therapy, although the mechanisms that control this resistance are largely unknown. Some studies have suggested that activation of the PI3K/mTOR signaling pathway may be responsible for trastuzumab resistance.
Read More »Anatomic Pathology Abstracts, 12/16
December 2016—Reproducibility of NEPTUNE descriptor-based scoring system with various types of images: The multicenter Nephrotic Syndrome Study Network, or NEPTUNE, digital pathology scoring system uses a novel and comprehensive methodology to document pathologic features from whole-slide images, immunofluorescence, and ultrastructural digital images.
Read More »Clinical Pathology Abstracts, 12/16
December 2016—Process optimization to improve immunosuppressant drug testing turnaround time: The routine use of immunosuppressant medications is critical for patients receiving solid organ transplants. Monitoring immunosuppressant (ISP) drug concentrations helps guide safe and effective dosing. ISP drug monitoring is performed using mass spectrometry or immunoassay methods.
Read More »Q&A column, 12/16
Q. Are there guidelines on microsatellite instability analysis by immunohistochemistry on colorectal adenocarcinomas? Specifically, should immunohistochemical stains for the mismatch repair enzymes be performed on all colorectal adenocarcinomas regardless of the clinical or pathological findings? A medical group recently requested these studies on all colorectal adenocarcinomas.
Read More »Newsbytes, 12/16
December 2016—The benefits of building a dedicated LIS support team: Too many cooks in the kitchen may be a problem, for which building more than one “kitchen” may be a solution. That’s the message Kathy Davis, manager of pathology informatics at the University of Michigan Medical Center, conveyed in an LIS management presentation at the 2016 Pathology Informatics Summit.
Read More »Put It on the Board, 12/16
SeraCare develops first multiplexed cardiomyopathy reference material: SeraCare Life Sciences has launched a multiplexed reference material for inherited disease testing by next-generation sequencing.
Read More »AMP case report: November 2016 test yourself answers
December 2016—In the November 2016 issue was a report, “Detection of cnLOH as a sole abnormality in the diagnosis of myelodysplastic syndrome,” written by members of the Association for Molecular Pathology. Here are answers (in bold) to the three “test yourself ” questions that followed that case report.
Read More »From the President’s Desk: Where we direct our gaze
December 2016—I gave a talk for the CAP16 House of Delegates in September that opened with a three-minute video showing how perspective can change along a continuum of distance. The clip depicts a logarithmic journey across space from a grassy field in downtown Chicago to the farthest reaches of outer space, then retraces and extends its path to the deepest interior (“inner space”?) of human cells. That would be mesmerizing even without the stirring—almost danceable—orchestral accompaniment. The overall effect is visually stunning and intellectually provocative. I have watched the video a number of times. It never fails to remind me that where we stand and how we focus our attention really do shape what we see and how we see it.
Read More »Sequencing goes deep to find rubella in uveitis patient
November 2016—Metagenomic deep sequencing, or MDS, has scored another coup in the diagnosis of an unexplained disease in a patient who had already had extensive workup with all other available tools. MDS had been used in 2014 to detect unsuspected leptospirosis in a critically ill encephalitis patient, enabling appropriate treatment and full recovery. Now some of the same clinical scientists at the University of California San Francisco Medical Center who helped diagnose that patient have identified rubella virus infection in the eyes of a patient with bilateral chronic intraocular uveitis that had been misdiagnosed as idiopathic inflammation for 16 years.
Read More »Big hopes, bigger questions with PD-L1
November 2016—Progress is a complicated minuet. One popular adage talks of “one step forward, two steps back,” which is not only discouraging but, in an even less-gleaming light, happens to be the title of one of Vladimir Lenin’s books, published in 1904. A more optimistic version (and one less centered on the crisis facing communists in turn-of-the-century Russia) suggests advances occur with two steps forward, mitigated by only one step back.
Read More »Up next for MALDI-TOF mass spec: AFB, molds
November 2016—Behold the humble API strip, made of plastic, with multiple miniature test chambers, interpreted with the aid of a color chart, and long a mainstay of microbiology laboratories.
Read More »Epi proColon fires up hopes of capturing screening dodgers
November 2016—When a Hollywood producer forecasts box office receipts, or a public health official contemplates action against a deadly but preventable cancer, there’s one hypothetical that might make both shudder: What if you held a screening and nobody came?
Read More »Who, what, when? Bringing order to influenza testing
November 2016—Raquel M. Martinez, PhD, D(ABMM), is very happy in her role as director of clinical and molecular microbiology at Geisinger Health System, Danville, Pa.
Read More »LIS of today a far cry from its ancestors
November 2016—When CAP TODAY magazine ran its first laboratory information systems product guide in the 1980s, an LIS was an LIS—nothing more and nothing less. Today, however, it’s a lot more, and the black and white of it have swirled to gray.
Read More »From the President’s Desk: When diagnosticians converse, 11/16
November 2016—This month’s column marks the midpoint of my term as CAP president. I have tried in the past year to write about topics that would foster discussion around certain underlying themes—most often communication, collaboration, and flexibility.
Read More »AMP case report: Detection of cnLOH as a sole abnormality in the diagnosis of myelodysplastic syndrome, November 2016
November 2016—Copy neutral loss of heterozygosity (cnLOH) is an acquired abnormality found in patients with cancer and hematologic disorders and can be detected by molecular techniques such as PCR-based analyses and hybridization-based chromosome genomic array testing (CGAT). We report a case in which cnLOH was the sole abnormality detected by CGAT in a patient with myelodysplastic syndrome. This case illuminates the importance of utilizing CGAT results, namely cnLOH findings, as one of the primary diagnostic indicators in order to expedite initial therapies.
Read More »Anatomic Pathology Abstracts, 11/16
November 2016—Relevance of papillary growth patterns of pulmonary adenocarcinoma, HPV involvement in head and neck cancers: assessment of biomarkers, Distinctive immunoregulatory microenvironment of medullary carcinoma of the colon, Diagnostic challenges caused by endoscopic biopsy of colonic polyps, MicroRNA expression profiling and expression of miR-205 in inflammatory breast cancer
Read More »Molecular Pathology Selected Abstracts, 11/16
November 2016—Misclassification of genetic variants associated with hypertrophic cardiomyopathy: Hypertrophic cardiomyopathy has a variable clinical presentation and may lead to sudden cardiac death. In many cases, it is associated with pathogenic genetic variants, enabling screening of relatives and, possibly, the ability to individualize treatment strategies through lifestyle modification or invasive procedures.
Read More »Clinical Pathology Abstracts, 11/16
November 2016—Neonatal ICU quality initiative: identifying preanalytical variables that contribute to specimen hemolysis: Hemolysis is a major cause of sample rejection and the need to recollect a specimen from a patient. In the neonatal intensive care unit, this may be of particular concern because of limited venous access and the risk of causing iatrogenic anemia.
Read More »Q&A column, 11/16
November 2016—As originally described, there are technically five Gleason patterns: 1, 2, 3, 4, 5. However, since patterns 1 and 2 are never used, there are no Gleason scores 1 + 1 = 2, 1 + 2 = 3, 2 + 1 = 3, 2 + 2 = 4, 2 + 3 = 5, and 3 + 2 = 5. Why is this? Isn’t this an alteration of Gleason’s original classification concept? Furthermore, there are cases in which a biopsy may contain a few glands that are diagnostic of carcinoma but insufficient to assign an accurate Gleason score. Would it simply be best to make a descriptive comment to that effect?
Read More »Newsbytes, 11/16
November 2016—How a pathology website can help build pathology websites: What if one could Google “biopsy orientation processing cassette” and receive detailed methodological instruction in surgical pathology as easily as finding a recipe for pound cake? Izak B. Dimenstein, MD, PhD, HT(ASCP), a grossing technologist retired from Loyola University Chicago Medical Center, believes highly specific information about laboratory methodologies should be easily accessible on the Web.
Read More »In memoriam: Tyra T. Hutchens, MD | 1921–2016
November 2016—Tyra T. Hutchens, MD, who served as CAP president from 1977 to 1979, died Aug. 28 at age 94. Dr. Hutchens was CAP vice president from 1975 to 1977 and a member of the Board of Governors from 1968 to 1974. He was a member of the Executive; Standards; and Budget, Planning and Review committees and of the Council on Government Relations and Liaison and the CAP History Editorial Board.
Read More »Shorts on Standards, 11/16
November 2016—The Laboratory Working Group of the National Kidney Disease Education Program and the IFCC Working Group for Standardization of Albumin in Urine are collaborating with IVD manufacturers to improve standardization of commercial measurement procedures. Urine albumin is an important biomarker for kidney damage.
Read More »Put It on the Board, 11/16
November 2016—FDA approves Ventana PD-L1 (SP142) assay, Access TSH (3rd IS) assay cleared for Beckman systems, Advanced MALDI-TOF use subject of AMP report, FDA clearance for Quidel Solana influenza A+B
Read More »AMP case report: October 2016 test yourself answers
November 2016—In the October 2016 issue was a report, “Laser capture microdissection: Vanishing roles in tissue microdissection revalued in salvaging a melanoma with micrometastasis for BRAF V600E mutation detection,” written by members of the Association for Molecular Pathology. Here are answers (in bold) to the three “test yourself ” questions that followed that case report.
Read More »Labs enter a MALDI-TOF state of mind
October 2016—When MALDI-TOF mass spectrometry enters the microbiology lab, it’s a little like watching Sir John Falstaff settle his considerable girth onstage. Things happen. Characters fret and flee, scheme, opine, panic, and, in the case of Prince Hal, ascend to greatness. (And, if we’re honest, some just get drunk.) Both, in brief, are an upending presence.
Read More »Blood bank: On guard against daratumumab interference
October 2016—As fans of spycraft know, offensive counterintelligence can include an arsenal of strategies: initiating a diversion, sowing confusion, creating false identities—anything that makes another party believe something that isn’t true. If the cancer treatment drug daratumumab were capable of deceptive intent, it might be accused of all those ploys when it comes to interfering with blood transfusion crossmatching. The reason: For patients receiving daratumumab, marketed as Darzalex by Janssen Pharmaceuticals, antibody testing for transfusion is subject to erratic false-positives, often leaving transfusion services confused, uncertain, and on hold.
Read More »Study finds what could be a key to prostate cancer progression
October 2016—The Gleason classification for prostate cancer is by no means going away. But within the Gleason grade, the presence or absence of a DNA-repair gene mutation may signal who is likely to proceed to invasive cancer, says Colin C. Pritchard, MD, PhD, lead author of a study published Aug. 4 in the New England Journal of Medicine.
Read More »Studies split on pre-prostate TRUS biopsy screening
October 2016—Take a bar graph, any bar graph, and compare it to the natural landscape of the United States. If most of it resembles the Great Plains but the right-hand side starts looking more like Rocky Mountain territory . . . well, something interesting is going on.
Read More »The President’s Desk: How clarity can bridge our silos, 10/16
October 2016—I had been looking forward to Cancer Biomarkers Conference II at Houston Methodist Hospital, even though early September wasn’t the best time to travel. Work was busy and CAP16 was only two weeks away. During the weekend meeting, I rediscovered the value of stepping back from business as usual. The program was fast-paced and stimulating, and I learned a lot. Even the nonpathologists I met clearly appreciated what I shared about how much pathologists do to facilitate the adoption of these new diagnostic tools.
Read More »Yale researchers dig for new kidney biomarkers
October 2016—An automated immunoassay has been created for symmetric dimethylarginine, or SDMA, a biomarker that can detect chronic kidney disease between 10 to 17 months earlier than creatinine, with 100 percent sensitivity and 91 percent specificity. And, unlike with creatinine, a patient’s muscle mass does not influence the biomarker’s reliability.
Read More »AMP case report: Laser capture microdissection: Vanishing roles in tissue microdissection revalued in salvaging a melanoma with micrometastasis for BRAF V600E mutation detection, October 2016
October 2016—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from the University of Florida College of Medicine, Jacksonville.
Read More »CAP16: All-star team presented with CAP and Foundation awards
October 2016—Gene N. Herbek, MD, was presented Sept. 25 with the Pathologist of the Year award during the spotlight event at the CAP16 annual meeting in Las Vegas. At the same event, Cordelia E. Sever, MD, was given the Pathology Advancement award, Sang Wu, MD, the CAP Foundation Gene and Jean Herbek Humanitarian award, Carey Zimmerman August, MD, the Outstanding Communicator award, and Denise K. Driscoll, MS, MT(ASCP)SBB, the CAP Staff Outstanding Achievement award.
Read More »When pain management testing calls for a consult
October 2016—Surprises might work for birthday parties—and even then they’re not everyone’s cup of tea—but not in drug screening programs. Perhaps the most common reason for doing a toxicology consultation is when a urine drug screen yields an unexpected result, either positive or negative, says Nicholas Heger, PhD, assistant director of clinical chemistry at Tufts Medical Center and assistant professor of anatomic and clinical pathology, Tufts University School of Medicine, Boston.
Read More »Anatomic Pathology Abstracts, 10/16
October 2016—Features of columnar-lined esophagus in gastroesophageal junction biopsies; Significance of Paneth cells in histologically unremarkable rectal mucosa; MicroRNA expression profile related to lymph node status in endometrial cancer; Plasma cells in melanoma: prognostic significance and possible role of IgA; Human epidermal growth factor receptor 2 testing in invasive breast cancer
Read More »Clinical Pathology Abstracts, 10/16
October 2016—Risk factors for transfusion in cesarean section deliveries at a tertiary hospital: Obstetrical hemorrhage is a major cause of morbidity and mortality in young women and may be difficult to predict. In some regions of the world, postpartum hemorrhage (PPH) may account for up to 25 percent of maternal deaths. Many studies have focused on the predictors of PPH before delivery.
Read More »Molecular Pathology Abstracts, 10/16
October 2016—Mutational landscape of glioblastoma under therapy: The mutational landscape of glioblastoma has emerged in recent years and includes frequently observed genomic alterations that are drivers of the tumor, including TP53, PTEN, EGFR, PIK3CA, ATRX, IDH1, PIK3R1, NF1, and PDGFRA.
Read More »Q&A column, 10/16
October 2016—What are the guidelines for proper handling and processing of blood specimens collected in serum separator tubes? Are there regulations guiding the practice of taking additional blood samples from a patient even though there are no orders for the blood samples? These “just in case” specimens are sent to our laboratory by the emergency department when a port or catheter is placed in the patient. The ED’s reasoning is that it prevents a patient from being stuck twice if there is an order for blood tests later. Our lab has to either store the samples or process them (centrifuge or separate RBCs from serum) so they are ready in case an order is entered later. Should this practice be banned? Should we refuse to accept these samples?
Read More »Newsbytes, 10/16
October 2016—Process improvement software more than online suggestion box; Viewics launches analytics tool for diabetes management; Hc1.com joins forces with Experian Health; OptraScan introduces whole slide imaging scanner; Contracts and installations
Read More »Put It on the Board
October 2016—Cancer Moonshot has diagnostic thrust: Vice president Joe Biden’s Cancer Moonshot now has a flight plan, drafted by a blue-ribbon panel and published in September. Coming as it does in the final year of president Obama’s term in office, there are doubts about whether the ambitious $1 billion program—aimed at achieving 10 years’ progress in cancer research and treatment in a five-year period—will ever get off the launching pad.
Read More »Beauty fad’s ugly downside: test interference
September 2016—It’s the kind of health promotion advice one might pick up casually over lunch with friends, in a quick Google search, or during a visit to the hairdresser. Take megadoses of an over-the-counter vitamin called biotin—a common supplement in multivitamin compounds—and watch your skin improve and your hair and nails thicken and gleam. In recent years, online social networks and health-related websites have begun to teem with ads claiming that people have seen a transformation since they jumped on the biotin bandwagon.
Read More »From the President’s Desk: In the eye of the brainstorm, 9/16
September 2016—Radiolab is a radio show and podcast about (mostly) scientific curiosities co-hosted by a perpetually interested guy who majored in music. You might describe it as a talk show for science geeks. These are people who know how to ask the right questions and put the answers in context. A recent installment (“Colors”) questions whether color is a concrete characteristic of the physical world or simply a mental overlay we apply to our perception. Early on, you learn that a young Isaac Newton pursued the mechanism of color perception by piercing his own eye with a knife. I was hooked.
Read More » CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management