Q. What is the current standard practice for collection tube order for CSF testing? Read answer. Q. What is the significance of the absence of coagulation of seminal fluid in a patient who previously experienced normal seminal fluid coagulation, followed by normal liquefaction, and had fathered children? Are there medications that can prevent seminal fluid coagulation? Read answer.
Read More »2018 Issues
Rapid PCR rules as labs ready flu arsenal
December 2018—With the memory of the 2017–2018 “high-severity” influenza season fresh in mind—49 million cases, 960,000 hospitalizations, a marginally effective vaccine, 79,000 deaths—clinical laboratories have been bracing for the customary annual surge in emergency room, outpatient clinic, and physician office influenza test orders. Although flu admissions have been rising somewhat, it is too soon to know how the season will play out, but laboratories are hoping for a season closer to average. Avoiding a repeat of last year’s travails—lengthy turnaround times, supply shortages, and the need to triage patients for testing—is a must, many laboratory directors say. “We had difficulty keeping up with last year’s demand. It was extremely time-consuming,” says Mary Kay O’Connor, national laboratory director at Summit Health Management, the management arm of the Summit Medical Group, an 800-provider practice on the East Coast.
Read More »Multiplex for allergy dx: powerful, but it has its place
December 2018—In allergy testing, microarray technology offers speed and the benefit of smaller sample volumes, but it has a lower sensitivity and is unable to detect IgE antibodies of all specificities in a given extract unless all allergens are on the chip. For routine use, singleplex assays are here to stay.
Read More »Could CGM dethrone HbA1c for office-based diabetes care?
December 2018—A glucose sensor the size of a quarter placed on the body and a sensor filament inserted under the skin could potentially disrupt traditional diabetes care with its continuous monitoring of glucose almost 300 times a day. Blood glucose can fluctuate widely during the day even in completely healthy people, said David Sacks, MB, ChB, in an interview with CAP TODAY.
Read More »Program zeroes in on histology, digital scan connection
December 2018—Quality in histology is at the heart of successful whole slide imaging, and a new program that rolls out in January will provide laboratories the aid they may need as they bring whole slide imaging on board.
Read More »Drug overdose deaths and toxicology tests: Let’s talk
December 2018—Drug overdose deaths in the United States continue to rise, and recently many of these deaths have been attributed to opioids, including fentanyl, fentanyl analogs, and other opioid receptor agonists. The rise in drug overdoses and drug-related deaths, and the devastating effects of the opioid crisis, highlight the need for communication and coordination among forensic pathologists, hospital clinicians, and laboratorians.
Read More »Higher pay for fibrinolysins interpretation in ’19 fee schedule
December 2018—The CMS finalized its 2019 Medicare physician fee schedule and its response to the CAP’s recommendations to raise payment for fibrinolysins interpretation and reporting and to forgo a proposed decrease to the physician work value for blood smear interpretation. The Centers for Medicare and Medicaid Services on Nov. 1 published the 2019 physician fee schedule. Services on the physician fee schedule are composed of three relative value units designated by the CMS: physician work, practice expense, and malpractice liability RVUs. Each RVU is separately valued and summed to equal the total RVU for each physician service on the fee schedule. The CAP advocates for the appropriate valuation of pathology services through its representation on the advisory committee of the AMA/Specialty Society Relative Value Scale Update Committee, known as RUC.
Read More »Urinalysis instrumentation: All eyes on standardized, scalable, integrated solutions
December 2018—On page 36 begins our urinalysis instrumentation product guide. The companies whose analyzers are profiled talked to CAP TODAY writer Valerie Neff Newitt
Read More »AMP case report: Discordant IHC/PCR test results for mismatch repair status in colorectal adenocarcinoma
December 2018—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Duke University Medical Center. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Read More »Put It on the Board
Pathologists and population health—
first steps
December 2018—Pathologists and population health—first steps: Pathologists who want to become involved in population health initiatives can take five main steps, say pathologists and laboratory leaders interviewed for an article published online last month in Archives of Pathology & Laboratory Medicine. In “The role of the pathologist in population health,” the authors report on the interviews they conducted and their review of the literature to answer several questions, among them whether pathologists in both large settings and smaller community-based settings can engage in population health (yes), and whether pathologists are in a position to analyze data for population health (“The data are there,” they say, “but getting to the data—and providing meaning out of it—is the hard part”). One of the first steps to becoming involved in any type of population health management activities, the authors write, is to understand the philosophy of the institution’s CEO and senior management.
Q&A column
Q. Can you explain further the revised CAP checklist requirement COM.40850 “LDT and Class I ASR Reporting,” which says to describe the method and performance characteristics in test reports unless the information is available to the clinician in an equivalent format? Read answer. Q. Can we see reactive lymphocytes in the pediatric population (under age two), and can we report them? Read answer.
Read More »Newsbytes
In molecular testing labs, gaps between actual and desirable LIS capabilities
December 2018—Flashback to 2013: Alexis B. Carter, MD, then director of pathology informatics at Emory University Hospital, was contemplating whether other pathology labs nationwide were facing the same challenges managing molecular testing data as she and her colleagues. So she decided to find out. Dr. Carter conducted a survey, and the responses confirmed her suspicions: Most laboratory information systems fall short in providing the infrastructure for complex molecular and genomic testing.
From the President’s Desk: Embracing our future at CAP18
December 2018—The CAP Curriculum Committee chaired by Sarah M. Bean, MD, follows a competency-based model to build an annual meeting program that is practical, prescient, and diverse. The committee is balanced demographically, experientially, and scientifically. Whatever the topic, there is someone who can speak to it.
Read More »Clinical pathology selected abstracts
Prostate cancer screening with PSA test: systematic review and meta-analysis
December 2018—Prostate cancer is the second most common cancer and the fifth leading cause of cancer-associated mortality among men worldwide. The use of serum prostate-specific antigen (PSA) to screen for prostate cancer is intended to detect the cancer at an early stage to reduce overall and disease-specific mortality. However, evidence that PSA screening for prostate cancer saves lives is somewhat lacking. Read More »Anatomic pathology selected abstracts
December 2018—Applying deep convolutional neural networks to diagnostic breast biopsies: The breast stromal microenvironment is a pivotal factor in breast cancer development, growth, and metastases. Although pathologists often detect morphologic changes in stroma by light microscopy, visual classification of such changes is subjective and nonquantitative, limiting its diagnostic utility.
Read More »Molecular pathology selected abstracts
December 2018—Profiling of chromatin-accessibility landscape of primary cancers: The chromatin-accessibility profiles generated in this study by The Cancer Genome Atlas represent the largest pan-cancer effort to characterize the regulatory landscape of human cancers. The study primarily used an assay for transposase-accessible chromatin using sequencing (ATAC-seq).
Read More »Time now for tumor mutational burden?
November 2018—Like a piece of so-called sticky music, cutoff numbers can persist in physicians’ minds outside of any real clinical value and, in the process, leave their laboratory colleagues mildly befuddled (not to mention searching for more useful cutoffs). Such a jingle is creeping into tumor mutational burden. Lauren Ritterhouse, MD, PhD.
Read More »New momentum for harmonizing lab results
November 2018—In The Music Man, the four members of the local school board, notorious for their squabbling, are assigned to investigate the credentials of “professor” Harold Hill, a charming con man who has come to town to sell band uniforms and instruments.
Read More »Biotin interference: answering questions, reducing the risk
November 2018—Biotin use is not rare, and don’t count on it being listed in the patient’s electronic medical record. Those are some of the findings of a Mayo Clinic study published recently in Clinical Biochemistry.
Read More »Component IgE testing offers food for thought
November 2018—Food component testing offers improved specificity for distinguishing IgE sensitized from truly allergic patients, and the menu for allergen components may soon expand.
Read More »AMP case report: Detection of concurrent hematologic malignancies in solid tumor NGS testing may cause false-positive results
November 2018—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Weill Cornell Medicine.
Read More »Inside the Color Atlas of Mycology: Candida famata
November 2018—Color Atlas of Mycology: An Illustrated Field Guide Based on Proficiency Testing is a new book from CAP Press, released in October. It is designed to help in identifying fungi using the most recent taxonomic classifications. In it is more than 15 years of proficiency testing data to highlight diagnostic clusters of incorrect identifications and address conceptual classification issues. Following is an excerpt from the section on yeast.
Read More »Laboratory information system vendors on where their focus is
November 2018—CAP TODAY’s lineup of laboratory information systems begins on page 41. Here, six of the 30 companies that have LISs listed in the guide tell us what you, our readers, want from your LIS and what they want you to know about them.
Read More »Hematology roundtable: rules, reference ranges, POC testing
November 2018—Reference intervals, point-of-care testing, the use of rules for efficiency, and the display of results in patient records. That and more is what a panel of experts weighed in on when CAP TODAY publisher Bob McGonnagle assembled them in September to talk about hematology instrumentation. What they told us follows.
Read More »On Roche m 511 analyzer, ‘everything is done from the slide’
November 2018—Roche Diagnostics will soon launch its m 511 analyzer for hematology laboratories. Krista Curcio, Roche technical marketing manager, hematology, told us, in a recent conversation with CAP TODAY publisher Bob McGonnagle, how and why the new instrument is different. “We’re turning it upside down and going a different way,” she said of the m 511. Here is more on the instrument Roche will launch before year’s end.
Read More »Put It on the Board
Quest acquires PhenoPath
November 2018—Quest Diagnostics has acquired PhenoPath Laboratories, which provides immunophenotyping, hematopathology, and molecular pathology services. The PhenoPath business, in Seattle, will operate as part of AmeriPath, a wholly owned business of Quest. Steve Rusckowski, Quest chairman, president, and CEO, said in a statement: “PhenoPath has a strong record of innovation and provides several capabilities that complement and extend our own, particularly in pathology and molecular oncology. It also deepens our presence in the Pacific Northwest.” PhenoPath founder Allen Gown, MD, tells CAP TODAY that continued consolidation in the laboratory industry and insurance reimbursement challenges have posed significant risks to PhenoPath’s future growth. “In Quest/AmeriPath,” he says, “we found an organization that realized not only the excellence of PhenoPath’s past and present but also the extraordinary future that, with their assistance, we can have.” Dr. Gown founded PhenoPath in 1998.
Q&A column
Q. Is anticoagulant adjustment in citrate tubes necessary when a patient’s hematocrit is less than 20 percent? Read answer. Q. What is the substitute test for HbA1c for a patient with homozygous variant hemoglobin? Is a fructosamine and/or glycated albumin test appropriate? Read answer.
Read More »Newsbytes
How Henry Ford core lab uses bottom-up communication November 2018—When Henry Ford Health System started planning its core laboratory’s automation line four years ago, aware that it needed to take Lean to the next level, it enlisted frontline laboratory employees in a development process that used the strategies of Hoshin Kanri and kaizen. Read more.
Read More »From the President’s Desk: Advocacy—Whose job is it anyway?
November 2018—Last month, we talked about how the CAP Laboratory Accreditation Program employs mentorship and perspective to move our specialty forward. I’d like to talk this month about how we apply those tools in our advocacy program.
Read More »Clinical pathology selected abstracts
Preparing for passage of regulatory requirements for laboratory-developed tests: November 2018—The FDA has raised concerns, in recent years, about several high-risk laboratory-developed tests (LDTs), including a concern that patients may undergo unnecessary treatment or delay or forego treatment due to the inaccuracy of such tests. Other agencies have also challenged the validity, accuracy, oversight, and safety of LDTs, a subset of IVDs that are intended for clinical use and designed, manufactured, and used within a single laboratory.
Read More »Anatomic pathology selected abstracts
November 2018—HER2: a pan-cancer event highly enriched in AR-driven breast tumors: Approximately one in five breast cancers is driven by amplification and overexpression of the HER2 receptor kinase, and HER2 enriched is one of four major transcriptional subtypes of breast cancer. The authors conducted a study to understand the genomics of HER2 amplification independent of subtype, as well as the underlying drivers and biology of HER2-enriched (HER2E) tumors.
Read More »Molecular pathology selected abstracts
November 2018—Common genetic variants contribute to risk of rare severe neurodevelopmental disorders: The traditional paradigm broadly classifies genetic diseases into rare disorders caused by a single gene variant and common disorders caused by complex interplay among multiple genes. However, recent research has shown that penetrance and disease phenotype, even in disorders thought to be monogenic, are affected by common genetic variation.
Read More »Letters: Ph-like ALL
November 2018—In the October issue of CAP TODAY, Karen Titus shared with us a story titled, “Fresh incentive to look for Ph-like ALL.” She spoke with key players in the discovery of BCR-ABL1-like B-lymphoblastic leukemia/lymphoma (B-ALL) (or “Ph-like” ALL), which is now a provisional entity in the 2016 revised fourth edition of the WHO. A key takeaway from the article ...
Read More »AMP case report: October 2018 test yourself answers
In the October 2018 issue was a case report (page 70), “NGS in the diagnosis of RASopathies in histologically uninformative skin biopsy samples,” written by members of the Association for Molecular Pathology. Here are answers (in bold) to the three “test yourself ” questions that followed that case report. 1. Which of the following is the most commonly mutated gene in ...
Read More »Fresh incentive to look for Ph-like ALL
October 2018—Cheryl Willman, MD, could hardly believe her eyes. She and her colleagues at the University of New Mexico, working with collaborators from across the U.S. in the NCI TARGET Project, had submitted 100 cases of high-risk pediatric acute lymphoblastic leukemia to British Columbia’s Cancer Agency for RNA sequencing to figure out why patients were doing so poorly, despite treatment with intensive chemotherapy.
Read More »Thyroid during pregnancy: how it changes, how to test
October 2018—How pregnancy affects normal thyroid function and thyroid function tests, and screening for thyroid disease during pregnancy, were the focus of a session at this year’s AACC annual meeting.
Read More »New requirements for use and storage of liquid nitrogen, dry ice
October 2018—Laboratory personnel safety is at the center of two new requirements and a revised requirement in the latest edition of the CAP accreditation program checklists, released in August.
Read More »Revenue cycle services: can they quell billing woes?
October 2018—Envision a long stretch of roadblocks of all shapes and sizes, staffed by unsympathetic and ever-changing guards, with new roadblocks springing up all the time, and you’ll probably have a fix on how most laboratories have viewed billing, going back decades.
Read More »New accreditation program checklist section: Imaging mass spec scores its own quality standards
October 2018—It happened for next-generation sequencing. It was an important step for in vivo microscopy. And now it’s taking place with imaging mass spectrometry. The milestone: development and adoption of a set of specialized checklist requirements for laboratories that want CAP accreditation. Imaging mass spectrometry, an adjunct methodology to help pathologists analyze areas of interest in tissue specimens, is, at this point, used in a small number of research laboratories in the U.S., says CAP Checklists Committee member Christopher M. Lehman, MD, clinical professor of pathology, University of Utah College of Medicine, and medical director of the University of Utah Hospital Laboratory.
Read More »New Color Atlas aids in identifying fungal species
October 2018—CAP Press released in October the Color Atlas of Mycology, by Gordon Love, MD, D(ABMM), and Julie Ribes, MD, PhD. Its 388 pages hold more than 800 tables and images, with identifications verified by DNA sequencing (for images post-2009). Here, in an exchange with CAP TODAY, Dr. Love explains how this atlas stands apart from others in the Color Atlas series and from others on the market.
Read More »Automated molecular platforms: 3 companies on what’s new and next
October 2018—CAP TODAY’s updated guide to the automated molecular platform market begins on page 45. Thirty-four platforms are profiled, with one new one: Hologic’s Panther Fusion. Writer Valerie Neff Newitt talked with three of the 20 companies about what they introduced this year, what’s to come, and more. “This is a dynamic and competitive industry. We are always asked to go faster, and that is what we are trying to do in terms of development,” says Michelle Tabb, PhD, chief scientific officer, DiaSorin Molecular. Others seem to be doing the same.
Read More »AMP case report: NGS in the diagnosis of RAS opathies in histologically uninformative skin biopsy samples
October 2018—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from the University of Washington, Seattle. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Read More »Shorts on Standards—Establishing cutoffs, reference ranges for biofluid biomarkers in Alzheimer’s disease: Reference materials and reference measurement procedures
October 2018—The newly developed National Institute on Aging and Alzheimer’s Association (NIA-AA) research framework uses a biological definition of Alzheimer’s disease.1 This framework has increased focus on biofluid biomarkers, especially because the measurement of cerebrospinal fluid amyloid beta peptide 42 (Aβ42) (or Aβ 42/40 ratio), phosphorylated tau protein (p-tau), and total tau proteins (T-tau) are included in the definition.1 The field of AD biofluid biomarkers is rapidly evolving. For example, CSF neurofilament light (NfL) is associated with AD neurodegeneration and may be a better CSF marker compared with T-tau.
Read More »Put It on the Board
Therascreen EGFR RGQ PCR kit approved as companion diagnostic for Vizimpro
October 2018—The FDA has approved a PMA supplement expanding the labeling claim of the Qiagen Therascreen EGFR RGQ PCR kit to allow its use as a companion diagnostic with Pfizer’s Vizimpro (dacomitinib). Vizimpro is for first-line treatment of patients with non-small cell lung cancer with EGFR exon 19 deletions or an exon 21 L858R mutation. The Therascreen EGFR RGQ PCR kit is now approved as a companion diagnostic to guide the use of three FDA-approved therapies, including also Gilotrif (afatinib) from Boehringer Ingelheim and Iressa (gefitinib) from AstraZeneca. It is registered in more than 40 countries. This was a project governed under an agreement between Qiagen and Pfizer.
Philips introduces computational pathology software for tumor detection
Royal Philips announced in September the latest release of TissueMark, which the company says now supports region of interest detection for the majority of molecular testing.
Q&A column
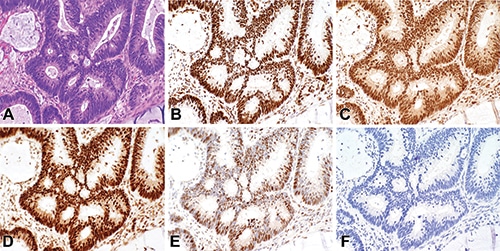
Q. How can one wisely apply GATA3 immunohistochemistry as a useful tumor marker in diagnostic surgical pathology? Read answer.
Read More »Newsbytes
Innovation labs: different means to the same end October 2018—What’s the best way for a hospital to kill a health care improvement-related idea? Some say (tongue in cheek) send it to committees, meetings, suggestion boxes, or the like. Read more.
Read More »From the President’s Desk—CAP accreditation: perspective-taking 101
October 2018—I grew up in the CAP as a volunteer in the Laboratory Accreditation Program. It’s a good place to dive in. Many of us do what I did—work our way through many volunteer opportunities over 30 years or more because each was so interesting. There are an amazing number of ways a person can approach challenges in a laboratory; more amazing is how many of the approaches will work. Partly that’s because the learning cuts both ways—I’ve learned as much when we were inspecting another laboratory as when my laboratory was being inspected.
Read More »Clinical pathology selected abstracts
RT-PCR detection of B. microti parasites using BMN antigens as amplification targets October 2018—Babesia microti infection, which is transmitted through the bite of an infected tick, is a growing health concern and continues to be a threat to the blood supply, with 22 states having reported cases of babesiosis in 2014. While most healthy adults with Babesia infection are asymptomatic or present with mild symptoms, including fever, fatigue, and anemia, babesiosis can be severe or fatal in neonates, the elderly, and immunosuppressed individuals.
Read More »Anatomic pathology selected abstracts
Analysis of ZC3H7B-BCOR high-grade endometrial stromal sarcomas October 2018—High-grade endometrial stromal sarcoma likely encompasses underrecognized tumors harboring genetic abnormalities besides YWHAE–NUTM2 fusion. Triggered by three initial endometrial stromal sarcomas with ZC3H7B–BCOR fusion characterized by high-grade morphology and aggressive clinical behavior, the authors investigated the clinicopathologic features of this genetic subset by expanding the analysis to 17 such tumors. All of the tumors occurred in women who were a median age of 54 (range, 28–71) years.
Read More »Molecular pathology selected abstracts
Cell-free DNA tumor mutational burden predicts efficacy of immune checkpoint inhibitors October 2018—Immune checkpoint inhibitors have emerged as a potent class of therapy for a variety of malignancies. The biologic rationale for these drugs is that somatic mutations, not necessarily in cancer driver genes, may accumulate in tumor cells, resulting in amino acid changes that create neoantigens (epitopes not present in normal cells during maturation of the immune system).
Read More »Letters
AMH immunoassays October 2018—In the article “Satisfaction high with new automated AMH assays” (June 2018), the focus seems to be on the presumed advantages of the Roche Elecsys and Beckman Coulter Access automated anti-müllerian hormone assays over manual AMH assays. The article reports that the main advantages of the automated platforms are less variability in AMH measurements, greater assay turnaround time, and greater assay cost-effectiveness.
Read More »Molecular ‘bucket list’ for renal cancer
September 2018—Leo Tolstoy is not listed as a coauthor on the most recent iteration of The Cancer Genome Atlas on renal cell carcinoma, which focuses on molecular characterization of RCC.
Read More »Hemophilia drug interferes with APTT-based assays
September 2018—When a miracle drug comes along that is predicted to cause havoc in the laboratory, the drug could well seem like a double-edged sword. In the case of emicizumab (Genentech’s Hemlibra), for patients with hemophilia A, the mix of both benefits and drawbacks is likely to settle in for the long term.
Read More »Forward march on commercial NAAT for M. genitalium
September 2018—About five years ago, when William M. Geisler, MD, MPH, was still focusing his research at the University of Alabama at Birmingham on chlamydia, Mycoplasma genitalium carried a lower profile as a cause of sexually transmitted infection.
Read More »For autopsy service, new requirements in AP checklist plus nine new requirements for forensic autopsies
September 2018—Quality management, communication, and consent are among the issues addressed in new and revised requirements in the autopsy pathology section of the latest edition of the CAP accreditation program anatomic pathology checklist.
Read More »From the President’s Desk: Policies to protect and preserve
September 2018—CAP leadership presents a gamut of responsibilities, including the enforcement of policies adopted to protect members and staff. What I am about to discuss is relevant to all organizations and work settings. As you read on, I hope you will reflect on how tolerance for inappropriate behavior could have an impact on your own workplace and what steps you can take to protect yourself, your colleagues, and by extension your patients.
Read More »Urine drug testing debate: How best to test compliance and manage opioid crisis
September 2018—Qualitative or quantitative testing. Hydrolyze or don’t hydrolyze. Use or don’t use standard cutoffs. These and other decisions in toxicology testing have taken on new urgency amid the opioid crisis, which is driving laboratories to change test methods to assess prescription drug compliance and illicit drug use.
Read More »‘We wanted to be the best we could possibly be’: CAP ISO 15189-accredited labs on the difference it makes
September 2018—Ten years ago, Richard J. Zarbo, MD, was feeling pretty proud of his laboratory. As system chairman of pathology and laboratory medicine at Detroit-based Henry Ford Health System, over the previous few years he’d seen his team rigorously implement Lean practices, practices that had paid off in greater safety and efficiency. “Setting the bar higher was important because that’s the culture here,” he says. “This is what we do.”
Read More »Next step? The switch from stool culture to PCR
September 2018—The advantages of moving from stool culture to a molecular platform are many: faster time to results, more accurate pathogen identification, a savings of space and staff time. For Jose Alexander, MD, D(ABMM), SM, MB(ASCP), and colleagues at Florida Hospital Orlando, another plus is being able to adhere to the Infectious Diseases Society of America guideline suggestion that labs use a diagnostic approach that can distinguish O157 from non-O157 E. coli and Shiga toxin 1 from Shiga toxin 2 E. coli.
Read More »Xifin CEO: Time to tune up negotiations with payers
September 2018—The second round of PAMA data collection is coming in 2019 and it’s critical to get it right, said Lâle White, CEO of Xifin, in a presentation in May at the Executive War College. If it’s not right, she warned, laboratories could see cuts that are more severe than those already seen.
Read More »Put It on the Board
Broad-based molecular testing for NSCLC September 2018—A recently published study on broad-based genomic sequencing and survival among patients with advanced non-small cell lung cancer in the community oncology setting should not lead to the conclusion that such sequencing should be avoided in nonsquamous NSCLC, say Paul A. Bunn Jr., MD, and Dara L. Aisner, MD, PhD, of the University of Colorado Denver, Aurora. Dr. Bunn, of the Department of Medical Oncology, and Dr. Aisner, of the Department of Pathology, in an editorial published Aug. 7 in JAMA, caution readers about the study published in the same issue, which found that broad-based sequencing (more than 30 cancer genes) directly informed treatment in a minority of patients and was not independently associated with better survival. The study of 5,688 patients with advanced NSCLC was based on data acquired through abstraction and aggregation of information from the electronic medical record from 191 U.S. community oncology practices.
Read More »Q&A column
Q. Is there expert advice or standard practice for releasing preliminary critical values for patients to the LIS pending subsequent technologist or technician verification and documentation? Read answer. Q. We hope to validate a procedure for the fixation, decalcification, and staining of bone marrow specimens but we will not be able to access fresh marrow specimens for our decalcification validation. Can you recommend an alternative tissue to validate the preservation of tissue morphology and antigenicity after decalcification? Read answer.
Read More »Clinical pathology selected abstracts
Outcomes of an audit of repeat lab testing at an academic medical center
September 2018—Overutilization of laboratory tests increases health care costs and may lead to false-positive test results and ambiguous findings. Unnecessary testing can result from a single order from a provider, an automated function in an order set, or a combination of the two.
Anatomic pathology selected abstracts
Thymoma: a clinicopathological correlation of surgical resection cases
September 2018—The authors presented 1,470 surgical resections for thymoma from the pathology files of 14 institutions in 11 countries with the purpose of determining and correlating a simplified histological classification of thymoma and pathological staging with clinical outcome.
Molecular pathology selected abstracts
Ability of genetic alterations to predict development of acute myeloid leukemia
September 2018—Acute myeloid leukemia affects more than 60,000 people in the United States every year and has a mortality rate of more than 90 percent. It is the most common form of acute leukemia and is caused by unchecked growth of immature precursor cells in the bone marrow. These immature cells, or blasts, are myeloid precursors that often develop into dysfunctional, cancerous white blood cells that fill the bone marrow and spread into the blood.
Newsbytes
Nebraska informaticians mine and translate genomic data
September 2018—More than five years have elapsed since clinicians at the University of Nebraska Medical Center approached the institution’s informatics department with a problem. They wanted to more easily access structured genomic data stored in the EHR system for the diagnosis of cancer patients.
Letters
Burnout
September 2018—As a pre-med student, I am shadowing a pathologist mentor at a major cancer hospital to gain insight into the life of physicians. She introduced me to CAP TODAY. I write in response to “Frontline dispatches from the burnout battle,” by Karen Titus (June 2018), to share the thoughts of a novice who now knows that becoming a physician means learning the skill of resiliency as early as possible.
Microbiology’s shifting role in war on sepsis
August 2018—If you were casting about for the severest test of a laboratory’s capabilities, day in and day out, sepsis admissions at a pediatric hospital might fit the bill. At Children’s Hospital of Philadelphia, and at other hospitals, waging war on sepsis requires battles on multiple fronts and clinical pathways that rely on an agile and highly equipped microbiology laboratory. Three main categories of patients ensure there is no shortage of sepsis cases at CHOP, says Erin H. Graf, PhD, D(ABMM), director of the infectious disease diagnostics laboratory.
Read More »Addressing the gender gap: Women and burnout—like men, but not
August 2018—Jennifer Hunt, MD, gave it her best shot. While picking up her son after school one day (a task she only rarely had time for) and hearing staff ask, “Who are you?” yet again …
Read More »Serial NT-proBNP found to identify risk for adverse CV outcomes
August 2018—For diabetes type 2 patients with cardiovascular disease, findings of a new study support clinicians’ use of serial measures of NT-proBNP concentrations to make critical treatment decisions easier by basing them on risk of major cardiovascular events, including heart failure.
Read More »PGx testing: recommended alleles for CYP2C19 panels
August 2018—After more than a year of gathering information and deliberating, members of the Association for Molecular Pathology Pharmacogenomics Working Group have issued the first in what will be a series of recommendations to standardize pharmacogenetic testing.
Read More »Transfusion medicine checklist: Record and other requirements updated in new release
August 2018—One new requirement and several modified requirements in the CAP transfusion medicine checklist are part of the new edition of CAP accreditation program checklists released this month. In work led by the CAP Council on Accreditation, the checklists are examined anew and revised yearly, where needed. In transfusion medicine, the changes this year center on computer crossmatches, record retention, forward/reverse typing, and ABO group and Rh(D) type verification.
Read More »Cytology workload limits: For adequacy assessments, it’s time, not slides
August 2018—The CAP and the Centers for Medicare and Medicaid Services reached an understanding earlier this year on how adequacy assessments and rapid on-site evaluations in cytology can be accounted for without causing undue impact on workload limits. The agreement, communicated to state survey agency directors in a March 16 CMS memorandum, is reflected in the updated CAP accreditation program cytopathology checklist released this month.
Read More »Molecular lung cancer testing: from guideline to practice
August 2018—Testing turnaround times can affect whether non-small cell lung cancer patients receive an EGFR or ALK tyrosine kinase inhibitor when indicated. At disease progression on an EGFR TKI, integrating circulating tumor DNA and tissue-based testing may lessen some of the limitations of each form of testing.
Read More »Cytopathology in Focus: Synergy in cytopathology and molecular microbiology
August 2018—In today’s less-is-more world, health care consumers and providers often seek explicit and detailed information from minimally invasive procedures and tiny samples. Over are the days of “malignant cells present” and on to the next case. Cytopathologists and cytotechnologists are embracing and integrating novel techniques and applying new methods to the diagnosis and classification of essentially every imaginable form of neoplasia. The 2018 WHO publications confirm that 29 percent of deaths worldwide (more than 10 million people annually) are attributable to communicable diseases.1,2 This means the purpose of procuring many specimens is not to just rule out malignancy but also to diagnose infectious etiologies.
Read More »Cytopathology in Focus: Why not call everything ASCUS?
August 2018—Below is a question shared on the ASC listserv. My reply to the question follows. A pathologist colleague who practiced previously as an obstetrician/gynecologist is of the opinion that categorizing the level of abnormality we observe on a Pap test is a waste of time. All the clinician needs to know, he says, is whether the test is normal or abnormal. The Pap test is a screening test, he says correctly, and its only relevance is in pointing out who needs a colposcopy and biopsy.
Read More »Cytopathology in Focus: Cytology social media—Facebook and Twitter as networking tools
August 2018—If you are not already using social media professionally, you may not know there is a vibrant and active community of pathologists, including many cytopathologists, on Facebook and Twitter—and getting involved is easy, fun, and educational.
Read More »Put It on the Board
Cobas HPV test approved for first-line screening using SurePath preservative fluid
August 2018—Roche received FDA approval for the Cobas HPV test to be used as the first-line screening test for cervical cancer in women 25 and older using specimens collected in SurePath preservative fluid. The Roche test is now the only HPV test approved for use as a primary screening test with both SurePath and ThinPrep PreservCyt Solution. It is approved for all of the screening indications supported by guidelines—primary screening in women 25 and older, reflex testing of unclear Pap test results in women 21 and older, and cotesting with a Pap test in women 30 and older—with both of the primary collection media types. “With this additional approval for the Cobas HPV Test, laboratories and clinicians now have an approved option that can be used for all of their HPV screening indications and sample types,” Ann Costello, head of Roche tissue diagnostics, said in a statement.
Q&A column
Q. What is the best way to report fecal fat testing? Read answer. Q. Who developed the formula for the corrected white blood cell count for nucleated red blood cells, and how was the formula established? Read answer.
Read More »From the President’s Desk: More influence in the AMA House
August 2018—This year, I made a point of noticing just how much goes on during the AMA Annual Meeting so I could tell you about it. There are 600 delegates now and an equal number of alternates. More special sections give us more ways to engage, and we do. It’s challenging, enjoyable, and exhausting at once.
Read More »Clinical Pathology Abstracts
Association of perioperative RBC transfusions with venous thromboembolism
August 2018—Hospital-associated venous thromboembolism is a major cause of morbidity and mortality, resulting in 100,000 to 200,000 deaths annually. Surgery can lead to a proinflammatory state and be a prothrombotic stimulus for venous thromboembolism (VTE). General anesthesia, as well as red blood cells transfused in the perioperative setting, is considered an independent risk factor for VTE.
Anatomic Pathology Abstracts
Analysis of the surveillance of women diagnosed with atypical ductal hyperplasia on core needle biopsy
August 2018—A needle core biopsy diagnosis of atypical ductal hyperplasia is an indication for open biopsy. The launch of randomized clinical trials of active surveillance for low-risk ductal carcinoma in situ leads to the paradoxical situation of women with low-grade ductal carcinoma in situ being observed and those with atypical ductal hyperplasia having surgery.
Molecular Pathology Abstracts
Prevalence of clonal hematopoiesis mutations in tumor-only clinical genomic profiling of solid tumors
August 2018—Challenges to implementing next-generation sequencing-based comprehensive molecular profiling of solid tumors include reliably separating germline variants from somatic variants. This is an important consideration, particularly when a “tumor-only” profiling approach is used.
Newsbytes
New digital pathology certificate program educates ‘from A to Z’
August 2018—While there’s a lot of buzz about the growth of digital pathology, its steep learning curve is a potential impediment to implementation. Recognizing this, the National Society for Histotechnology, in collaboration with the Digital Pathology Association, launched in May a web-based digital pathology certificate program designed to provide both a broad overview of digital pathology and a deep dive into the details.
Small groups, big answers in HER2 testing
July 2018—Take the new ASCO/CAP guideline for HER2 testing. Since the first groundbreaking joint guideline appeared 11 years ago, the authors have made a habit of addressing cases that flummox pathologists, medical oncologists, and patients. Now, in 2018, they have clarified the diagnostic approach to in situ hybridization groups two, three, and four, rare cases that nonetheless cause an outsized share of headaches and worries. It also clarifies language from the 2013 guideline that had sent some labs astray, and it addresses the use of multiple alternative chromosome 17 probe assays. The previous guidelines turned out to be tough acts to follow—a bit like following Sean Connery in the role of James Bond—even as the new one benefits from new data.
Read More »Look, wait, buy: labs share instrument plans
July 2018—“Robbie,” the autonomous service robot that transfers specimens for Florida Hospital’s central laboratory, may not quite be ready for his gold watch. But after five years of faithful service delivering samples between the different esoteric testing units, he’s nearing the end of his natural lifespan with signs of wear.
Read More »With CMS coverage policy, NGS cancer testing goes large
July 2018—The March 16 announcement of a new Centers for Medicare and Medicaid Services coverage policy for next-generation-sequencing–based diagnostic lab tests for patients with advanced cancer did not appear out of the blue, since a draft policy was issued last fall.
Read More »Artificial intelligence: what’s possible, why now?
July 2018—When it comes to artificial intelligence, it can be difficult to distinguish hyperbole from reality. So how much can AI truly replace human tasks in society and, more specifically, in medicine?
Read More »In memoriam: Harold H. Harrison, MD, PhD (1951–2018)
July 2018—Harold H. Harrison, MD, PhD, 67, Pennsylvania state commissioner in the CAP Laboratory Accreditation Program and a member of the Inspection Process Committee, died suddenly June 6 of cardiac causes. Dr. Harrison joined the Geisinger Health System in Danville, Pa., in 2007 where he was director of clinical pathology and director of Geisinger Regional Laboratories.
Read More »For pain care and more, PGx testing at Avera Health
July 2018—Putting pharmacogenetic testing into play at Avera Health was years in the making. It took time to operationalize it at an affordable cost. Today, it has wide physician acceptance and is seen as a strong benefit for patients. “Pharmacogenetics is what will differentiate Avera in a new era of ACOs and personalized medicine, and will ultimately lead to a model for transforming health care,” says Trisha Lauterbach, MS, MLS(ASCP)CM, laboratory operations manager at Avera Institute for Human Genetics (AIHG), Sioux Falls, SD.
Read More »Direct oral anticoagulants and APTT, PT results: The risk of normal results in patients on therapy
July 2018—With the introduction of direct oral anticoagulants (DOAC) there is a paradigm shift in the use and understanding of screening coagulation tests to determine a patient’s bleeding risk. In patients on DOAC therapy, clinicians cannot rely on normal activated partial thromboplastin time (APTT) and prothrombin time (PT) results to reflect the patient’s level of anticoagulation.
Read More »Menu, security, consistency: vendors point to priorities
July 2018—Chemistry and immunoassay analyzers combined—that’s what is new about the product guide. In years past, the chemistry and immunoassay analyzer product guides were published separately. This year we integrated them and are publishing them in two issues.
Read More »Put It on the Board
High-sensitivity troponin I assay available in the U.S.
July 2018—Beckman Coulter Diagnostics received 510(k) clearance from the Food and Drug Administration for its new high-sensitivity troponin assay, Access hsTnI, for use on the Access 2, DxI, and the entire Access family of immunoassay systems. Access hsTnI demonstrates less than 10 percent CV at the upper reference limits for men and women and detects troponin in more than 50 percent of the healthy population. In an independent study, Access hsTnI detected more than 99 percent of troponin values for healthy men and women (Pretorius CJ, et al. Clin Biochem. 2018;55:49–55). “Beckman Coulter’s high-sensitivity cardiac troponin I assay can measure very low cardiac troponin concentrations with excellent precision. This test may help physicians with both the early diagnosis of myocardial infarction and future risk stratification in and outside the acute coronary syndrome setting,” Peter Kavsak, PhD, associate professor, Department of Pathology and Molecular Medicine, McMaster University, said in a statement.
Clinical Pathology Abstracts
Trends in perioperative RBC transfusion from index cases in five surgical specialties
July 2018—In recent years, greater attention has been given to patient blood management. While contemporary national guidelines recommend restrictive red blood cell transfusion, it is not known whether such transfusions have decreased in surgical patients. Approximately 11 million RBC transfusions are performed annually, and two-thirds of those are for patients in the perioperative period.
Q&A column
July 2018—Is CD30 currently being used as a predictive marker for therapy? Due to laboratory construction, our molecular instruments were relocated within the lab. Is full test validation required in this case? Or is running at least 20 known samples enough to verify the instrument/assay performance specifications?
Read More »President’s Desk: From concept to fruition
July 2018—CAP members know that laboratory quality improvement and accreditation drive much of what we do. Nobody can know everything about the science underlying our specialty because pathology embraces a vast body of knowledge that is always changing …
Read More »Newsbytes
July 2018—How a geospacial tracking system is closing the distance at Michigan lab: This summer, when the Michigan Medicine Department of Pathology moves the last of its laboratories to a new campus four-and-a-half miles from the existing University Hospital laboratories in Ann Arbor, it will kick its new PathTrack Lean-influenced real-time geospatial tracking system into high gear. Developing the system, which will monitor an anticipated 6,000 to 10,000 specimens every day, has been “a monumental effort,” says Ulysses J. Balis, MD, director of the health system’s division of pathology informatics.
Read More »Anatomic pathology Abstracts
Clinical and molecular analyses of neuroendocrine carcinomas of breast
July 2018—Clinical and molecular analyses of neuroendocrine carcinomas of breast: Neuroendocrine breast carcinomas represent a rare subtype of breast cancer. Their definition, prevalence, and prognosis remain controversial, as reported in the literature.
Molecular pathology selected abstracts
July 2018—Correlation between tumor mutation burden and efficacy of combination immunotherapy in nonsmall cell lung cancer: Checkpoint inhibitor therapy has dramatically improved outcomes in many cancer types, with treatments including antibodies against cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), programmed cell death receptor-1 (PD-1), and its ligand (PD-L1).
Read More »Letters
July 2018—I was excited to see the article “Clearing the air for electronic cancer checklists” (May 2018) and would like to share what we at Sunquest are doing to contribute to adoption of the electronic cancer checklists. Sunquest is continually improving the PowerPath Synoptics tool set, which is included as part of our base system.
Read More »Frontline dispatches from the burnout battle
June 2018—Bryan Bohman, MD, doesn’t spend his days wandering the Bay Area handing out buttons that read “Lift people, not the bottom line.” But don’t rule this out as a possibility someday, either. Dr. Bohman, chief medical officer, University Healthcare Alliance, and clinical professor of anesthesiology and perioperative and pain medicine, Stanford Health Care, is campaigning against physician burnout. Yes, it threatens the quality of medical care, he says, and yes, it’s expensive.
Read More »For one laboratory, a workflow transformation
June 2018—“Form follows function” is a famous design principle, coined by an architect. But in the health care system and elsewhere, perfect matches between form and function are scarce.
Read More »NGS to take top spot as cancer biomarker testing broadens
June 2018—For biomarker testing and tissue conservation, all roads lead to next-generation sequencing, says Boaz Kurtis, MD, laboratory and medical director of Cancer Genetics in Los Angeles. Dr. Kurtis said, “There’s no other technology platform out there that can provide the amount of data we need today or will need in the future.”
Read More »Questions about frozen tissue, preanalytic variables, tumor content
June 2018—Boaz Kurtis, MD, laboratory and medical director of Cancer Genetics in Los Angeles, took the following questions from attendees at a webinar on NGS in routine non-small cell lung cancer biomarker testing.
Read More »Satisfaction high with new automated AMH assays
June 2018—Testing for anti-müllerian hormone got a boost in 2017 when the year began and ended with the FDA clearing the first two fully automated AMH assays from Roche and Beckman Coulter.
Read More »Core needle biopsy of the breast: cases and cautions
June 2018—With core needle biopsies of the breast, if something looks like an epithelial malignancy, ask yourself: Is it really a carcinoma? If it is a carcinoma, ask yourself if it is a primary breast carcinoma.
Read More »Instrument acquisition, skilled labor on the table: Makers of chemistry, immunoassay analyzers answer our questions
June 2018—Broad menus, efficient workflows, single platforms, rural labs, cybersecurity, and economics are some of what one CEO and three marketing and product managers talked to CAP TODAY about when we spoke to four companies whose analyzers are profiled in the product guide.
Read More »New reference guide for ultrasound-guided FNA
June 2018—CAP Press released last month a new reference guide, Ultrasound Features of Superficial and Palpable Lesions. It’s small and spiral bound and has a laminated cover and tabs for easy reference. There are 375 images and illustrations in the guide’s 200 pages.
Read More »Q&A column
June 2018—What is the role of total testosterone and free testosterone in gauging the effectiveness of androgen deprivation therapy?
We are planning to validate the mismatch repair panel in our immunohistochemistry laboratory. Do we use the CAP guidelines for antibody validation for a nonpredictive marker or a predictive marker?
Clinical Pathology Abstracts
June 2018—Multiple myeloma and precursor disease in firefighters at World Trade Center disaster; Association between time to colonoscopy after a positive fecal test and colorectal cancer
Read More »Anatomic Pathology Abstracts
June 2018—Impact of pattern of invasion in invasive endocervical adenocarcinoma; Inflammatory myofibroblastic tumor of the uterus: an analysis of 13 cases.
Read More »Molecular Pathology Abstracts
June 2018—Genetics and pathogenesis of diffuse large B-cell lymphoma: Understanding the genetic basis of diffuse large B-cell lymphoma is important for understanding the pathogenesis of the disease and the molecular attributes that may influence therapeutic response.
Read More »Newsbytes
June 2018—Electronic device shows promise for identifying pathology specimens: While barcodes and radio-frequency identification are considered the workhorses of pathology specimen identification, a new technology, nearly two decades in the making, may soon get a piece of the action.
Read More »Put It on the Board
June 2018—FDA clears T2Bacteria panel for detecting sepsis-causing pathogens: T2 Biosystems received market clearance from the Food and Drug Administration for the T2Bacteria panel for the direct detection of bacterial species in human whole blood specimens from patients with suspected bloodstream infections.
Read More »Letters
June 2018—LCIS variants and DCIS: We write in response to the article by Karen Lusky regarding tips to distinguish DCIS from variant forms of LCIS (April 2018). A different question might be: Is it actually important to distinguish these two in situ proliferations?
Read More »From the President’s Desk—CAP18: bringing it all back home
June 2018—Nobody has an annual meeting like ours. Nobody. And CAP18 will be no exception. The CAP18 educational program features 85 courses presented by 128 outstanding faculty. A quick scan of the program reveals 42 new learning opportunities and another 38 returning by popular demand.
Read More »Skirting the pitfalls of merging lab results
May 2018—“One of these things is not like the others” is a fun puzzle for kids in the context of Sesame Street. But it can be a vexing informatics challenge when you are managing data entered in fields in a database. For anyone charged with merging outside laboratory results into an institution’s electronic health record alongside results from an in-house laboratory, the differences can generate no end of headaches.
Read More »New contender speeds ID with susceptibility testing
May 2018—If a seismic shift were to happen in microbiology, the technology behind the Accelerate Pheno system and PhenoTest BC test kit, which won FDA approval last year as a rapid pathogen identification and antimicrobial susceptibility testing (AST) system for blood cultures, could well be the cause.
Read More »Teaming up: how one site is managing its complex liver cases
May 2018—It didn’t take long for Heather Stevenson-Lerner, MD, PhD, to grasp one key fact about the liver biopsy cases she was seeing at the University of Texas Medical Branch, Galveston: They were often complicated. UTMB sees plenty of challenging liver cases of its own, says Dr. Stevenson-Lerner, assistant professor of medicine and liver and transplantation pathologist, Department of Pathology.
Read More »Pharmacogenomics advocates make case for wider use
May 2018—Use of pharmacogenomic testing is still limited, despite ample research, the existence of guidelines, and the emerging evidence it can help patients. Panels can be costly and insurance coverage variable, and providers need guidance—from pharmacists, the lab, decision support alerts—in knowing what and when to order and in understanding the results. Plus, patients move.
Read More »From the President's Desk: What we learn from member surveys
May 2018—As a professional society, we want to know what our members need so we can provide services and programs to help them excel. We know that pathology practices are diverse because they have to be—science is never static. We also know that practice settings vary widely. In short, CAP members’ interests and concerns are uncommonly diverse because our field is uncommonly diverse.
Read More »Clearing the air for electronic cancer checklists
May 2018—Length, cost, variability in vendor support, and lack of consistency have cast a cloud for pathologist users over the CAP’s cancer protocols and the electronic version of those protocols, the electronic cancer checklists. Work is underway to improve the user experience (Nakhleh RE, et al. Arch Pathol Lab Med. 2017;141[9]:1153–1154). Behind that effort is the undeniable: “Structured discrete data, using a controlled vocabulary, can be captured, stored, and reviewed much more readily than data in other formats,” says Mary Edgerton, MD, PhD, vice chair of the CAP’s Pathology Electronic Reporting (PERT) Committee and associate professor of pathology, University of Texas MD Anderson Cancer Center.
Read More »With hemolysis, tackling the rush with the reasoning
May 2018—First a journey. Then sometimes a vigorous shake. Little wonder that red blood cells hemolyze. “Red blood cells don’t like to be stressed,” says Kathleen Finnegan, MT(ASCP)SH, phlebotomy training program director at Stony Brook University School of Technology and Management, New York. She instructs her students to avoid stressing the RBCs by skipping what she calls the “martini shake” (CLSI recommends five to 10 tube inversions), using a needle that is the right size, and not using a syringe for transfer but instead a transfer device. “So it’s gentle,” she says.
Read More »Cytopathology in Focus: Reporting salivary gland cytopathology—new user-friendly Milan system consists of six diagnostic categories
May 2018—The Milan System for Reporting Salivary Gland Cytopathology was published Jan. 31 and is an important step toward standardizing the reporting of salivary gland fine needle aspiration. A large body of literature has demonstrated that FNA is an effective method for the initial evaluation of salivary gland masses, but until this year there was no uniform, widely accepted reporting system. The complexity of salivary gland cytology poses unique challenges that demand a standardized approach to communication of diagnostic information between pathologists and treating clinicians.
Read More »Cytopathology in Focus: For thyroid cytopathology, the 2017 Bethesda System
May 2018—Surgical pathologists take their tumor nomenclature from the WHO Classification of Tumours, but cytopathologists take their terminology from where the consensus groups convened—Bethesda, Paris, Milan, and Yokohama—to formulate terminology recommendations. The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC)1 is now in its second edition.
Read More »Clinical Pathology Abstracts, 5/18
May 2018—Use of a smartphone app to assess neonatal jaundice: Neonates are screened for hyperbilirubinemia before hospital discharge using a transcutaneous or total serum bilirubin measurement. However, levels peak at approximately 96 hours of life, which is after most healthy infants have left the hospital. Outpatient follow-up is often performed by visual inspection, but this can be highly variable and have poor interobserver agreement.
Read More »Anatomic Pathology Abstracts, 5/18
May 2018—Outcomes related to use of hygroscopic sonographically detectable clips: The use of hygroscopic sonographically detectable clips (HSDCs) has dramatically increased in recent years, especially for breast cancer patients who undergo neoadjuvant chemotherapy. The authors conducted a study to define the appearance of HSDC sites in histopathological specimens and allow pathologists to recognize these sites and differentiate them from other lesions.
Read More »Molecular Pathology Abstracts, 5/18
May 2018—DNA methylation-based testing to classify central nervous system tumors: Despite being the mainstay of pathology tissue diagnostics, microscope-based histological review has limitations. Among them is that pathologists may have differing opinions about a case. This interobserver variability may result in over- or undertreatment of the patient and lack of agreement about which diagnosis is correct.
Read More »Newsbytes, 5/18
May 2018—Vendor neutral archives: A fit for the pathology lab? Whether the initialism VNA will become as recognizable as the acronym PACS in the pathology field remains to be seen, but pathologists and vendors alike are considering how vendor neutral archives may benefit the pathology lab.
Read More »Q&A column, 5/18
May 2018—Our immunohistochemistry laboratory is moving to a new building across the street. We are not getting new equipment, just moving the machines to the new building. Do we need to perform a full revalidation of all our antibodies?
Read More »Put It on the Board, 5/18
May 2018—FDA approves osimertinib for NSCLC, nivolumab + ipilimumab for RCC: The FDA on April 18 approved osimertinib (Tagrisso, AstraZeneca) for the first-line treatment of patients with metastatic non-small cell lung cancer whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations, as detected by an FDA-approved test.
Read More »Q&A column
April 2018—A semen analysis for viability was collected at 9:30 AM and not received in the laboratory until 1:40 PM. Our standard operating procedure says this test must be analyzed one hour after collection, with no disclaimers stated for late receivables. Therefore, it is my understanding that a specimen received five hours after collection would be considered unacceptable because the viability of the semen is compromised and the collection delivery does not follow our SOP.
Read More »TB testing: new approaches to old scourge
April 2018—Scratch the surface of TB testing, and things quickly get interesting. The standard skin reaction test, widely adopted by the early 1940s, is still in use today. The goal has remained steady as well: break the transmission cycle. “From the clinician perspective and the laboratory perspective, because of its infectious nature, we want to identify people with latent tuberculosis,” says Elitza Theel, PhD, lab director for the infectious disease serology laboratory, Mayo Clinic and Mayo Medical Laboratories.
Read More »HBsAg tests, mutation in public health spotlight
April 2018—If you were asked to pick a place on the map where problems with detecting a mutant strain of an infectious disease would likely come to light, the capital of Nebraska might not be your first guess.
Read More »Digital pathology: A 1st anniversary report card
April 2018—Nearly one year after the FDA cleared the Philips IntelliSite Pathology Solution for primary diagnosis, Philips is reporting worldwide momentum for the adoption of digital pathology.
Read More »Fewer false-positive pregnancy results with intact hCG
April 2018—When women of childbearing age check in at a cancer center where they might be undergoing medical or surgical treatment, the screening protocol is often to test them for pregnancy, primarily by quantifying serum β-hCG.
Read More »From the President’s Desk: Pathologists as medicine’s first responders
April 2018—The National Institute on Drug Abuse estimates that 100 million Americans suffer from chronic pain. The majority of drug overdose deaths involve an opioid, and nearly half of drug overdoses caused by opioids involve prescription drugs.
Read More »LCIS variants and DCIS: tips on telling them apart
April 2018—DCIS or LCIS? Making the distinction can be difficult in some cases. Stuart J. Schnitt, MD, in a session at CAP17 on ancillary testing in breast pathology, delineated the reasons and provided tips, including the role of E-cadherin immunostains to help in this distinction. The cells of DCIS typically show strong membrane staining for E-cadherin while the cells of LCIS are typically E-cadherin negative. But among the tips: If an in situ lesion is E-cadherin positive, it doesn’t automatically mean it’s ductal carcinoma in situ. As he demonstrated in several cases, the lesion could be lobular carcinoma in situ with aberrant E-cadherin immunostaining.
Read More »Turnover in phlebotomy: looking deeper than pay
April 2018—Laboratory managers struggling to reduce turnover among phlebotomists should look beyond the pay and examine their hiring and management practices and the dysfunction that could be creating walls between analytical and preanalytical staff. “It’s an enormous problem,” Dennis J. Ernst, MT(ASCP), NCPT(NCCT), director of the Center for Phlebotomy Education, says of phlebotomist turnover. “There’s no silver bullet because there are so many things that lead phlebotomists to give up hope where they work and in the profession. It’s critical that managers are tuned in to the needs of this specialized workforce because they’re varied and many.”
Read More »POC glucose: views on volume, critical care, ACOs
April 2018—Test volume, limitations on devices used in critical care, consolidation, and population health is what CAP TODAY asked about when it spoke in March with the makers of three bedside glucose testing systems. Their systems and those of two other companies are profiled on pages 44-49. “The customers are more aware than ever of the limitations that are in the package inserts from the glucose manufacturers,” says Corrine Fantz, PhD.
Read More »In cervical disease dx, agreement rises with p16 IHC use
April 2018—In analyzing cervical tissue, adjunctive use of p16 IHC with H&E-stained slides improves accuracy and sensitivity, according to the results of the Cervical Tissue Adjunctive Analysis study presented by Thomas C. Wright Jr., MD, in a webinar hosted by CAP TODAY and made possible by an educational grant from Roche Diagnostics.
Read More »Clinical Pathology Abstracts
April 2018—Potential contributors to error in oxygen saturation calculation using a POC assay: Oxygen saturation is important for measuring respiratory status and calculating cardiac output for patients.
Read More »Anatomic Pathology Abstracts
April 2018—Confirmation of ProMisE: a clinical classifier for endometrial cancer; Leiomyoma with bizarre nuclei: a morphological, IHC, and molecular analysis.
Read More »Molecular Pathology Abstracts
April 2018—Effect of inherited TP53 mutations on children with B-cell ALL: TP53 has been referred to as the “guardian of the genome” because it plays a central role in regulation of the cell cycle, DNA repair, and apoptosis, and because somatic mutations in TP53 are frequently identified in many tumor types.
Read More »Newsbytes, 4/18
April 2018—Data-extraction system demonstrates potential for pathology laboratories: Just as parents instill in their children a desire to improve themselves, in part through interactions with others, some software developers are “teaching” their tools to interact and adjust accordingly.
Read More »Put It on the Board, 4/18
April 2018—CMS changes proposed policy on NGS for cancer patients: The CAP and the Association for Molecular Pathology, in separate statements in March, lauded the Centers for Medicare and Medicaid Services for revising its national coverage determination on next-generation sequencing.
Read More »Letters, 4/18
April 2018—Let us bring back breast FNAB: The article “Standardized reporting for breast FNAB cytology” (January 2018) is a welcome account of some of the challenges associated with breast fine needle aspiration biopsy compared with core needle biopsy. The author expertly outlines the significance of a standardized approach to performance, interpretation, and reporting of the breast FNAB procedure. The article was a pleasant surprise because the topic of breast FNAB has not been the subject of much interest in our pathology community during the past several years.
Read More »Lung guideline goals: more tests, treatment
March 2018—Among the many never-ending chores that humans undertake—paying bills, filing taxes, flossing—writing medical guidelines can seem like an especially perpetual task. Just ask the architects of an updated document on molecular testing for lung cancer, issued by the CAP, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology.
Read More »Labs take stock of surprising flu season
March 2018—In a severe flu season that started early, laboratories faced unprecedented test volumes, used new testing platforms, and negotiated vendor supply shortages. When laboratory staff at Arkansas Children’s Hospital in Little Rock began seeing a rising number of requests for respiratory tests, and five positive flu results,
Read More »Puzzling out the positive shift in the final 14-day rule
March 2018—When the CMS’ new 14-day rule took effect Jan. 1, conditions for laboratories doing outpatient reference testing might have changed for the better. But for labs navigating the new billing regulations, some forecasters are predicting confused seas ahead. “We’ve been reaching out to a number of our customers who I know will be affected by this and saying ‘What’s your take?’ and together just putting our heads around what it really means. But there is still quite a bit of confusion out there,” says Kurt Matthes, vice president, reengineering and service, at revenue cycle management software provider Telcor.
Read More »Inflammatory biomarkers foreshadow CKD, study finds
March 2018—The central idea of the film Minority Report—that a “precrime” police unit can predict and prevent crimes—still mostly inhabits the realm of science fiction. Luckily, in medicine, researchers studying “predisease” can make headway on prevention by analyzing the laboratory test results from samples collected years earlier, when patients showed no clinical symptoms, that might have been able to predict disorders such as chronic kidney disease (CKD) in those patients.
Read More »From the President’s Desk: A warm welcome around the world
March 2018—I was just out of training and still getting my bearings when I learned that the new partner in our group was expected to manage CAP Laboratory Accreditation Program inspections. Today I think that is a good idea, but back then I was nervous. I had done several CAP inspections as a resident. But did I know enough to get a laboratory through one?
Read More »Why carbapenemase-producing CRE raise the bar
March 2018—When Stephen Brecher, PhD, compares MRSA to carbapenem-resistant Enterobacteriaceae, the figures of speech come fast and furious. “We are not in Kansas anymore,” he said. “The bar has been raised. I consider MRSA a picnic compared to CP-CRE [carbapenemase-producing CRE].”
Read More »Pros and cons of carbapenemase detection tests
March 2018—When it comes to diagnostic tests, everyone wants the same thing Lars Westblade, PhD, wants: A unicorn. “The diagnostic performance of a test is reflected in its sensitivity and specificity,” Dr. Westblade said. “It has to be a very good test. And then we need to think about the speed of the test.” There’s also the cost. When all these factors come together just so, “we get what’s called diagnostic perfection,” he says, or the rare event that Brandi Limbago, PhD, of the CDC calls “a diagnostic unicorn.”
Read More »For AP signout: infectious diseases pathology atlas
March 2018—New from CAP Press this month is the Atlas of Fundamental Infectious Diseases Histopathology: A Guide for Daily Practice, edited by Bobbi S. Pritt, MD, MSc, DTM&H. It covers bacterial, viral, fungal, and parasitic infections and contains more than 800 images.
Read More »Gene testing moves cardiomyopathy analysis forward
March 2018—From phenotype to genotype in the understanding and diagnosis of cardiovascular disease—that was the medical journey on which Joseph Maleszewski, MD, and Birgit Funke, PhD, took attendees at a symposium at the November 2017 meeting of the Association for Molecular Pathology.
Read More »Clinical Pathology Abstracts, 3/18
March 2018—Web platform vs. genetic counselor for releasing carrier results from exome sequencing: Genomics can be used to generate a large amount of data that may have important implications for clinical care and selection of therapeutics. However, a bottleneck exists in clinical genomics due to the large volume of results and the lack of availability of knowledgeable professionals to return them to patients in person.
Read More »Anatomic Pathology Abstracts, 3/18
March 2018—Magee equation 3 for predicting response to chemotherapy in some breast tumors: Magee equations were derived as an inexpensive, rapid alternative to the Oncotype DX commercial assay. Magee equation 3 uses immunohistochemical and FISH data for estrogen receptor (ER), progesterone receptor (PR), HER2, and Ki-67 for its calculation: 24.30812+ERIHC×(–.02177)+PRIHC×(−0.02884)+(0 for HER2 negative, 1.46495 for equivocal, 12.75525 for HER2 positive)+Ki-67×0.18649.
Read More »Molecular Pathology Abstracts, 3/18
March 2018—Nonendoscopic detection of Barrett’s esophagus using DNA methylation biomarkers: Esophageal adenocarcinoma is an aggressive disease, with a less than 20 percent five-year survival rate, and its incidence is rapidly increasing. Early detection of esophageal adenocarcinoma or its precursor lesion, Barrett’s esophagus, would enable more effective treatment strategies and a greater chance of cure.
Read More »Newsbytes, 3/18
March 2018—How hospitals use savvy and software as a phishing net: We all know we shouldn’t click on suspicious emails, but suppose you see an email from your department of human resources with an attached document about a new dress code. You open it, thinking “What new dress code?” And now you’ve infected the hospital’s computer system with a virus.
Read More »Q&A column, 3/18
March 2018—Our pathology group has an unusual case of residual squamous cell carcinoma of the lung in a lobectomy specimen after chemotherapy. The lung shows a hilar scar (1.7 cm) involving the lung parenchyma and the peribronchial adipose tissue. In the scar there is residual carcinoma (0.4 cm) that focally is involving the peribronchiolar adipose tissue around the lobar bronchus. The focus is located at 0.3 cm of the final surgical resection margin of the bronchus. Because the tumor involves peribronchiolar adipose tissue, is it considered outside the lung (extension outside the lung)? Since the tumor is in the mediastinal fat around the bronchi and had to invade the viscera pleura to invade the peribronchial adipose tissue, would the tumor stage be ypT2a? Or T3 since it is invading part of the mediastinal fat? Or should it be pT1?
Read More »Put It on the Board, 3/18
March 2018—AMP issues recommendations for clinical CYP2C19 genotyping allele selection: To promote standardized testing across laboratories, the Association for Molecular Pathology published on Feb. 27 consensus, evidence-based recommendations for designing and validating clinical CYP2C19 assays.
Read More »New HPV guideline for head, neck cancers
February 2018—Like a pair of one-size-fits-all jeans, testing all head and neck carcinomas for human papillomavirus may have seemed like a good idea at one time. In many cases, in fact, HPV testing is just what treating clinicians and patients need. On the other hand, not every head and neck case requires it. Now, a newly published CAP guideline should help physicians figure out the right fit in multiple settings.
Read More »Scoring gastric, GEJ cancers for PD-L1 expression
February 2018—To some ears, perhaps, the scientific method connotes a process that is standardized and unimaginative. But inventions like Velcro, vulcanization, and the microwave—all stemming from accidental discoveries—testify to the role of luck and leaps of intuition in formulating and modifying a hypothesis.
Read More »Molecular tumor board: a patient with ALK– rearranged lung cancer
February 2018—A case of ALK-rearranged lung cancer was the subject of a multidisciplinary molecular tumor board presented last fall at CAP17 by pathologist Laura J. Tafe, MD, and oncologist Benjamin Levy, MD. Together they offered up insights into the tumor genomics of lung cancer with talk of testing guidelines, targeted therapies, resistance mechanisms, and circulating tumor DNA analysis.
Read More »Smart test ordering—new program provides the tools
February 2018—A new CAP program with a novel approach makes it easier to take on an old problem: misapplied laboratory tests. The CAP Test Ordering Program, available now and complimentary to all members, is different from other laboratory test utilization initiatives, says Richard W. Brown, MD, medical director for system laboratory services at Memorial Hermann Health System in Houston.
Read More »From the President’s Desk: Human factors engineering
Last month we talked about the eighth edition of the American Joint Committee on Cancer staging manual, which was launched Jan. 1. CAP experts had undertaken a full-court press to harmonize our cancer protocols with the new edition.
Read More »AMP case report: Detection of rare deletion mutation in the alpha-globin gene locus establishes a diagnosis of Hb H disease
February 2018—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Quest Diagnostics. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Read More »Procedures up to date? Fighting injury in phlebotomy
February 2018—Requiring strict adherence to the latest industry standard for venipuncture can go a long way to minimizing the risk of phlebotomy-related lawsuits and multimillion-dollar jury awards. “It revolves right back to education,” says Nancy Erickson, PBT(ASCP), an expert witness in more than 30 phlebotomy-related lawsuits. She says lack of education and failure to follow the standard of care cause the two most common patient complaints that lead to phlebotomy-related litigation: nerve damage and syncope.
Read More »AP-LIS vendors talk reports, interfaces, protocols
February 2018—Customer demand, cancer protocols, and consolidation of pathology practices are some of what CAP TODAY asked about when it spoke in January with four anatomic pathology computer system companies. Their AP systems and those of 17 other companies are profiled in the anatomic pathology computer systems interactive product guide. “It’s a really good time for our market right now,” says Joe Nollar of Xifin, “and systems providers need to be creative in helping their clients get the solutions they need to be scalable, competitive, and profitable.” Here is more of what they told writer Anne Ford.
Read More »For precision medicine, next-generation mass spec
February 2018—The modern analytical technologies of mass spectrometry continue to garner prominence and broader utility in clinical diagnostics. This was showcased at the 7th Annual American Association for Clinical Chemistry Conference on Mass Spectrometry and Separation Sciences for Laboratory Medicine, held last fall in Philadelphia. Representatives of academia, industry, and regulatory bodies came together to share information about the technology and best practices, the aim of which is to strengthen clinical diagnostics for the betterment of patient care. In opening remarks, then CAP president Richard Friedberg, MD, PhD, shared his hopes for the future of mass spectrometry in anatomic pathology.
Read More »Clinical Pathology Abstracts, 2/18
February 2018—Restrictive or liberal approach to red blood cell transfusion for cardiac surgery: Among the largest group of recipients of red blood cell transfusions are patients undergoing cardiac surgery. Whether a restrictive approach to intraoperative and postoperative transfusion in cardiac surgery is superior to a more liberal approach with regard to patient outcomes is unclear.
Read More »Anatomic Pathology Abstracts, 2/18
February 2018—ARTEMIS trial: quantifying pathological response to neoadjuvant chemotherapy: The Affordability and Real-World Antiplatelet Treatment Effectiveness after Myocardial Infarction Study, also known as the ARTEMIS trial, tested standard neoadjuvant chemotherapy with or without bevacizumab in the treatment of HER2-negative early breast cancer.
Read More »Molecular Pathology Abstracts, 2/18
February 2018—Gene expression and risk of leukemic transformation in myelodysplasia: The myelodysplastic syndromes represent a group of clonal hematopoietic disorders with varying prognoses, with survival ranging from a few months to more than 10 years. Multiple laboratory measurements have been used in attempts to provide reliable prognostic assessments, including bone marrow blast counts, severity of peripheral cytopenia, cytogenetic findings, and, most recently, gene-mutation profiling.
Read More »Newsbytes, 2/18
February 2018—The many facets of a laboratory IT budget: Creating an information technology budget for the laboratory may seem like a fairly straightforward, if painstaking, exercise. But a number of factors that can affect the laboratory’s bottom line are frequently overlooked during the budgeting process, according to two health care consultants who spoke with cap today.
Read More »Q&A column, 2/18
February 2018—I come from a core (hematology/chemistry) background, and I would like practical, how-to guidance in developing an effective QC strategy for HIV viral load testing. What performance characteristics do you verify? How many and what type of samples do you use? What are the chosen acceptable thresholds? Do you use L-J charts? If so, what do you plot, what control rules do you select, and how do you select them?
Read More »Put It on the Board, 2/18
February 2018—PreludeDx unveils predictive assay for DCIS: PreludeDx announced results from its oral presentation at the San Antonio Breast Cancer Symposium. The results using the SweDCIS randomized trial confirmed that the DCISionRT test predicts which patients with ductal carcinoma in situ will benefit from radiation therapy after breast-conserving surgery.
Read More »Clin Lab 2.0: Add value, make patients better
January 2018—It was baseball’s Yogi Berra who said, with the unique slant that was his hallmark, “In theory there is no difference between theory and practice. In practice, there is.” More vividly, boxer Mike Tyson once summed up the same reality when asked to comment on an opponent’s strategy in an upcoming match: “Everybody has a plan—until they get hit.”
Read More »Genotype-guided dosing of warfarin: GIFT wrap-up
January 2018—In an ideal world, clinical research data would be applied with immediate and beneficial effect to clinical practice, especially when the data come from a well-controlled, well-run trial that meets the gold standard of being large, randomized, and blinded.
Read More »Next-gen sequencing finds further clinical utility in oncology
January 2018—One of the plenary sessions at the 2017 meeting of the Association for Molecular Pathology—“High Impact Molecular Diagnostics for Cancer and Inherited Diseases”—was a virtual mini-course in the latest and most useful applications of next-generation sequencing to detect germline and somatic mutations in cancer. Both speakers zeroed in on the clinical utility of their innovative diagnostic techniques.
Read More »New Color Atlas of Hematology to be used ‘in the wild’
January 2018—New this month from CAP Press is the second edition of the Color Atlas of Hematology: An Illustrated Field Guide Based on Proficiency Testing. “More and better are the watchwords,” senior editor Eric F. Glassy, MD, told CAP TODAY when we asked what the reader can expect. David Blomberg, MD, and Katherine Galagan, MD, are associate editors.
Read More »From the President’s Desk: A shared vision for cancer staging
January 2018—For me, friendships formed and lessons learned have more than compensated for the effort invested over the years on CAP committees, but make no mistake: When we meet, what we’re doing is work. The professional engagement is enjoyable, but a person can get tired toward the end of a two-day meeting, not to mention homework in the evenings.
Read More »Devices, decisions: POC glucose in the critically ill
January 2018—Using point-of-care glucose meters in critically ill patients can feel like tiptoeing through a regulatory minefield. Perhaps your preferred meter hasn’t been cleared by the FDA for use in this population. Or maybe you’re not sure which assay performance requirements should be regulating the performance of your meters. Or perhaps you’re still trying to define “critically ill.”
Read More »Lab needs driving coagulation analyzer market
January 2018—Customer wish lists help to define every generation of coagulation analyzers, test menus, and related technologies. That’s evident in the recent and upcoming launches and the ongoing work of the companies whose analyzers are profiled in this issue in the 2018 coagulation analyzer product guide.
Read More »AABB seeks comments on form to streamline transfusion adverse reaction reporting
January 2018—The AABB is seeking comments by March 30 on its common transfusion reaction reporting form, the seven pages of which are presented online at www.bit.ly/AABB-reportform. The fillable PDF form is intended to be used by hospitals and blood centers to communicate information about transfusion reactions to the blood supplier, particularly when there are multiple suppliers to the hospital transfusion service.
Read More »Cytopathology in Focus: Standardized reporting for breast FNAB cytology
January 2018—In countries with developed medical infrastructure, the use of breast fine-needle aspiration biopsy (FNAB) cytology has had its share of challenges over the past 20 years, among them the use of core needle biopsies. In developing countries where the use of FNAB cytology has been increasing rapidly, breast lesions are one of the most common sites sampled by FNAB. In 2016, the International Academy of Cytology Executive Council put together a “Breast Group,” which consists of cytopathologists, surgical pathologists, radiologists, surgeons, and oncologists working in breast care, with the aim of producing a comprehensive and standardized approach to breast FNAB cytology reporting.
Read More »Cytopathology in Focus: A right and a wrong way to use CAP educational kits
January 2018—The CAP Cytopathology Committee constructs educational and interlaboratory comparison kits that are distributed regularly to cytotechnologists, cytopathologists, and pathologists who want continuing education in cytopathology. The purpose of the kits is to make it possible for those who screen and diagnose cytology slides to maintain and update their skills. However, the Cytopathology Committee has been made aware that the kits have been employed for purposes other than education. We address here the potentially detrimental uses to which some laboratories are putting these educational kits and advise laboratories to use them only as they were intended.
Read More »Cytopathology in Focus: Pap proficiency testing: what’s permitted, what’s not
January 2018—Annual proficiency testing is mandated for all cytotechnologists and pathologists who sign out Pap tests. CAP staff frequently receive questions about the PT rules or hear of situations in which there was confusion about the rules, a few of which we will highlight here.
Read More »Cytopathology in Focus: HPV vaccines: the decade in review
January 2018—Diane Harper, MD, MPH, and Leslie DeMars, MD, provide an extensive review of the efficacy of available FDA-approved HPV vaccines in different age groups and describe immunogenicity findings in particular (Gynecol Oncol. 2017;146:196–204). The World Health Organization and the Centers for Disease Control and Prevention recommend a two-dose vaccine for younger children due to high rates of seroconversion and antibody titers in this age group. Girls age 15 and older should continue to get three doses.
Read More »Anatomic Pathology Abstracts, 1/18
January 2018—Analysis of desmoplastic pattern at the tumor front in colorectal cancer subtypes: Although recent findings of cancer biology research indicate that prognostic power arises from genes expressed by stromal cells rather than epithelial cells, desmoplastic reaction has not been completely examined as a prognostic marker for colorectal cancer. The authors conducted a pathologic review of 821 stage II and III patients who underwent R0 resection for colorectal cancer at four independent institutions.
Read More »Clinical Pathology Abstracts, 1/18
January 2018—Drone transport of chemistry and hematology samples over long distances: Interest in using unmanned aerial vehicles, or drones, to transport laboratory specimens is based on the need to move specimens from satellite facilities to a central hub for testing. Earlier studies of biological specimens transported by drones were performed in ambient or cold temperatures for a maximum flight length of 40 minutes.
Read More »Molecular Pathology Abstracts, 1/18
January 2018—Importance of interstitial genes that exist between gene fusion partners in prostate cancer: Prostate cancer is one of the most common cancers affecting men, yet understanding of the disease’s development and progression is limited. One of the most effective ways to stratify treatment and outcomes is based on pathology review of prostate biopsies, though the application of molecular testing to these samples is increasing.
Read More »Newsbytes, 1/18
January 2018—Why pathologists shouldn’t ‘pass the baton’ with IT: It may be tempting to stay in your comfort zone and leave the technology decisions to the information technology experts. But pathologists who abdicate oversight of IT projects within their departments are setting up those projects for failure, says John H. Sinard, MD, PhD, professor of pathology and medical director of pathology informatics at Yale University School of Medicine.
Read More »Q&A column, 1/18
January 2018—We are in the process of validating the Stago STA Compact Max and Stago STA R Max with cap piercing. The company is stating that the open and closed modes follow the same testing pathway and therefore validation between modes is not necessary. Is this correct? Is PHI (phosphohexose isomerase), also known as GPI (glucose phosphate isomerase), mainly responsible for metastasis and circulating tumor cells?
Read More »Put It on the Board, 1/18
January 2018—Some safety issues more gray than black and white: Most laboratory safety rules are free from ambiguity. Anyone who handles specimens must wear personal protective equipment, for example. Some issues are less clearly defined, though, and require a deeper dive into the guidelines. That is where Dan Scungio, MT (ASCP), SLS, CQA(ASQ), comes in.
Read More » CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management