Amy Carpenter Aquino
September 2018—Qualitative or quantitative testing. Hydrolyze or don’t hydrolyze. Use or don’t use standard cutoffs. These and other decisions in toxicology testing have taken on new urgency amid the opioid crisis, which is driving laboratories to change test methods to assess prescription drug compliance and illicit drug use.
“From a provider’s perspective, it’s difficult to provide adequate pain control while avoiding the risk for abuse,” said Athena Petrides, PhD, director of toxicology and assistant director of chemistry at Brigham and Women’s Hospital, in a session at the AACC annual meeting in July. Dr. Petrides, who is also an assistant professor of pathology at Harvard Medical School, told attendees that more than 40 percent of chronic pain patients report feeling inadequately treated for pain.
At Brigham and Women’s Hospital, the laboratory responded to the increased demand for pain toxicology testing—which rose from approximately 750 tests in 2005 to approximately 3,500 tests in 2017—by developing a new assay to assess pain medication compliance.
“It is straight-to-LC-MS/MS definitive testing. We eliminated the immunoassay,” said co-speaker Stacy Melanson, MD, PhD, associate director of clinical laboratories and co-director of chemistry at Brigham and Women’s Hospital. “There are several guidelines, including one from us as laboratory scientists, that support mass spectrometry as the best methodology to assess medication compliance.”
The new assay is the result of the laboratory’s efforts to adapt urine drug testing to provide more guidance to clinicians and to help manage the opioid crisis, said Dr. Melanson, an associate professor of pathology at Harvard Medical School. “We’ve had a journey with urine drug testing and many changes” coinciding with the opioid crisis.
The speakers framed their AACC session as a friendly back-and-forth discussion on best practices for assessing prescription opioid compliance and illicit drug use. Dr. Petrides took the stance that urine is the preferred specimen for testing while Dr. Melanson defended oral fluid as the specimen of choice. The speakers also argued the merits of quantitative versus qualitative testing, hydrolysis, and standard cutoff limits. “These are all issues that are up for debate,” Dr. Petrides said.
Recent guidelines recommend urine drug testing as a risk assessment tool to detect the presence of prescribed medication as evidence of regimen adherence and to identify unauthorized substances, Dr. Petrides said. “Urine is considered to be the specimen type of choice due to the wider window of detection and the noninvasive way to collect a specimen,” she said, adding: “Oral fluid, however, is increasingly discussed as an alternative to urine.”
The stakes have never been higher for those who manage chronic pain patients. CDC data show that between 59,000 and 65,000 drug overdose deaths occurred in 2016, outnumbering peak deaths from car crashes, HIV, or firearms in any other year, Dr. Petrides said. At least half of all opioid-related overdose deaths reported in 2016 involved a prescription opioid.
One bright spot in the CDC’s prescription opioid overdose data—the decrease in prescription opioid medication rates between 2006 and 2016—was overshadowed by the increase in average days of supply from 13 in 2006 to 18 in 2016, an overall increase of 35.7 percent. “So that means that patients now have more pills in their house,” Dr. Petrides said.
The laboratory at Brigham and Women’s detected significant levels of prescription opioid diversion and illicit drug abuse in its patient population. “We saw 14.7 percent of patients that were potentially diverting their medication, and 28.7 percent of patients were positive for non-prescribed or illicit drugs,” Dr. Petrides said. The synthetic opioid fentanyl is a growing concern. U.S. Drug Enforcement Agency data showed more than 30,000 fentanyl seizures in 2016, almost double the number in 2015, she said. “We’re getting a high positivity rate for fentanyl in our patient population.”

Dr. Petrides
Requests for pain toxicology panels from pain management physicians at Brigham and Women’s declined in recent years while requests from non-pain management physicians were on the rise. “Anecdotally, what we’ve heard is that testing is shifting to the primary care physicians because they see the patient more frequently and are taking over their management,” she said. The clinicians who regularly order pain toxicology tests and interpret the results are “no longer the population we had at the beginning of this epidemic.” Clinicians with less experience in interpreting pain toxicology results may require more guidance from the laboratory.
The previous toxicology panel offered at Brigham and Women’s was a hybrid, Dr. Petrides said. Amphetamines, buprenorphine, cocaine metabolite, fentanyl, THC, barbiturates, tramadol, 6-acetylmorphine, EDDP, and methadone were tested by immunoassay, which offered a large test menu and fast turnaround time.
The platform’s advantages did not overcome its challenges when testing for benzodiazepines and opioids. The lack of specificity in the immunoassay for these classes of drugs in particular required definitive testing by liquid chromatography tandem mass spectrometry (LC-MS/MS). “We were doing immunoassay for some drugs and sending out the positive results to a reference laboratory for confirmation and upfront definitive testing for opioids and benzodiazepines,” Dr. Petrides said.
Data showed a high false-positive rate for many drugs screened by immunoassay, specifically for 6-acetylmorphine, amphetamines, fentanyl, and buprenorphine. “We had an issue,” she said.
The laboratory’s solution was to “create one big definitive testing panel” and test for all prescribed and illicit substances in-house by mass spectrometry. The panel reflected a compromise between the speakers’ positions on the critical components of a definitive testing panel, which Dr. Petrides described as quantitative versus qualitative testing, hydrolysis, and cutoff levels.
“For the sake of this debate, I am going to support reporting qualitative results, performing the hydrolysis step, and using standard cutoffs,” Dr. Petrides said, noting that the views she and Dr. Melanson were going to express during the session were for debate purposes only.
Asked if the test results could be used to determine whether the patient was taking her medication as prescribed, most in the session said no. Dr. Petrides agreed. “Urine drug testing has many variables to determine what the measured drug response is going to be,” she said. “Drug interactions is one; you could be competing for binding to enzymes with these drugs, so the metabolism of that drug isn’t happening as rapidly as you would expect.” Adherence, which is the question in this case, is another.
Other factors to consider: urine adulteration caused by drinking large amounts of water to dilute the urine and mask the presence of the drug; genetic variation if the patient has alterations in the cytochrome P450 enzymes or glucuronosyltransferases (UGT) that cause a different metabolic pattern or conjugation of the drugs; a clinical diagnosis, such as a kidney or liver disease, which could affect results; dose management; and the half-life of the drug—“the most important thing here.”
“I think qualitative testing is the way to go in this case to avoid confusion,” Dr. Petrides said. “You want to avoid the misinterpretation and assumption that you can use these results to determine dose and timing, which is what the physicians are trying to do.” If the question is one of compliance, “why do you need those quantitative numbers? Isn’t the fact that the drug is there evidence enough?”
Qualitative testing requires fewer calibrators, and assay validation is less laborious, she added.
Dr. Petrides made the case for performing the hydrolysis step, which involves incubating the sample to remove the glucuronide group, and sometimes the sulfate group, in order to measure total drug levels instead of the different metabolites—conjugated metabolites—and the primary drug. “You increase your detection sensitivity because you have a larger pool now and it avoids false-negatives,” she said.
Adding the hydrolysis step also results in fewer drugs and their metabolites in the testing panel. “If you have all the results listed for the different glucuronides, it’s going to be a gigantic list and it will be very difficult for clinicians to go through and read every single one,” she said. By using a panel with appropriate metabolites, “you will make interpretation for clinicians easier and allow them to better assess compliance.”
 In the past, for example, noroxycodone was not included in their panel. “It was difficult to assess whether the oxymorphone result was due to oxycodone ingestion or oxymorphone ingestion. It can be either one. By having noroxycodone, you’re able to differentiate that,” Dr. Petrides said.
In the past, for example, noroxycodone was not included in their panel. “It was difficult to assess whether the oxymorphone result was due to oxycodone ingestion or oxymorphone ingestion. It can be either one. By having noroxycodone, you’re able to differentiate that,” Dr. Petrides said.
With oxycodone and oxymorphone in the panel, if the patient is prescribed oxycodone, “in our patient population that would be 31 percent of the positive results.” Adding noroxycodone raised the positivity rate to 36 percent, “and you’re definitely sure that that comes from oxycodone metabolism.”
“This is showing the confidence in your interpretation.”
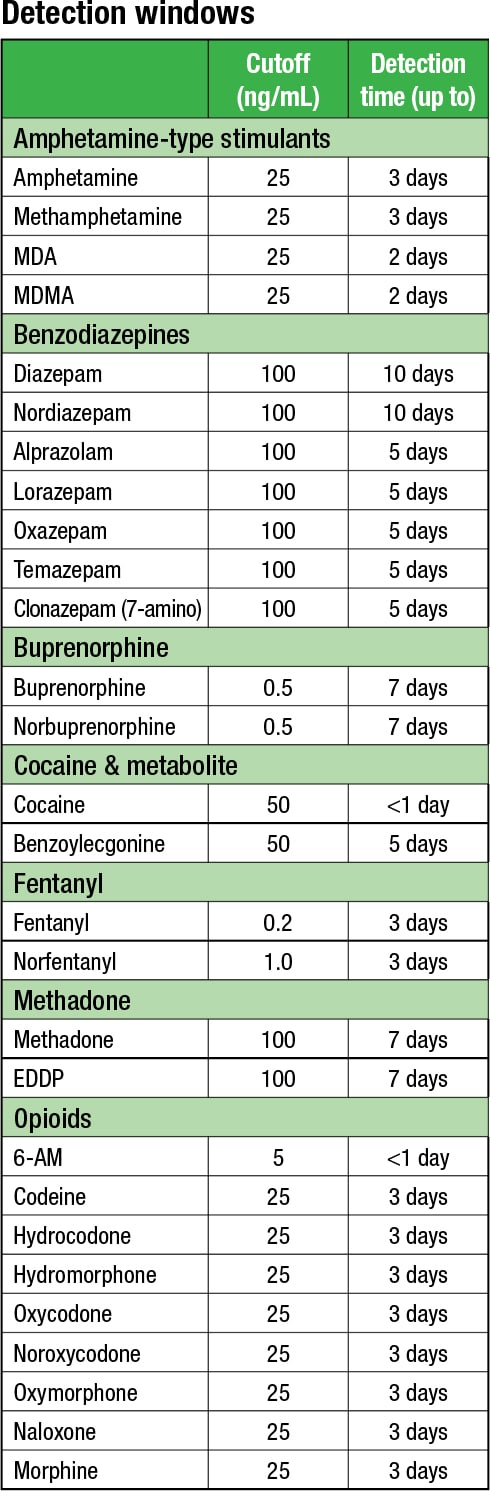
Dr. Petrides shared another example from her laboratory to support the use of standard cutoffs. “We have an assay that detects benzoylecgonine down to 5 ng/mL—you can go that low—and we had a positive result. The clinician called us and said, ‘My patient is adamantly saying they did not take cocaine. Please send it to another lab.’” Brigham and Women’s sent the specimen to another lab, which had a cutoff of 50 ng/mL, and the result came back negative. “That causes some suspicion of how accurate our results are in our lab. If we all use standard cutoffs, we can consistently assure clinicians that these methods are actually valid for testing their population.”
Scientific data exist defining detection windows with use of standard cutoffs, she said. “Although that can vary between individuals, at least you have a ballpark of when the patient might have taken this drug,” which helps the laboratory answer a common question from providers: Was the cocaine strongly or weakly positive? “We can’t answer those questions if we have very, very low cutoffs that we don’t have any data around.”
Laboratories with standard cutoffs would be able to use the accepted detection windows for the different types of drugs, Dr. Petrides said.
Dr. Melanson argued that laboratories should report results quantitatively, not hydrolyze, and use the lower limit of quantitation.She presented the case of a 54-year-old chronic pain patient with a history of substance abuse managed with methadone. The provider ordered the pain toxicology panel to assess compliance, and results showed that methadone and EDDP (methadone metabolite) were detected, reported qualitatively. Urine creatinine was normal. “If you had those results and your clinician called, would you say this patient is compliant?” Dr. Melanson agreed with the majority of session attendees who answered yes.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
