 December 2018—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Duke University Medical Center. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
December 2018—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Duke University Medical Center. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Jenna McCracken, MD, PhD
Jadee Neff, MD, PhD
Mismatch repair (MMR) status testing is recommended for all patients with colorectal carcinoma to identify those at risk for Lynch syndrome, inform disease prognosis, and guide therapeutic management.1,2 Germline mutations in MMR genes and consequent microsatellite instability (MSI) are hallmarks of Lynch syndrome, an autosomal dominant disorder associated with a high risk of developing colorectal, endometrial, ovarian, and hepatobiliary tract cancers. Patients and family members with Lynch syndrome benefit from increased cancer surveillance and more radical surgery.1 Ten to 15 percent of sporadic colorectal cancers are also MMR deficient due to MLH1 hypermethylation and are notable for BRAF mutations.3,4 Regardless of germline status, all patients with defective MMR have a better overall prognosis with fewer liver and lymph node metastases.1,5 Tumors with defective MMR are less responsive to 5-fluorouracil–based chemotherapy and more sensitive to the immunomodulatory drug pembrolizumab.6,7 For these reasons, it is now generally accepted that all patients with newly diagnosed colorectal cancer should be screened for MMR status, a strategy endorsed by the National Comprehensive Cancer Network.8
Microsatellites are tandemly repeated DNA sequences distributed throughout the genome that, due to their redundant nature, are particularly prone to slippage and duplication/deletion errors during DNA replication. The stability of microsatellites is dependent upon functional MMR proteins. The two most common MMR testing modalities are complementary in nature: an immunohistochemical assay for the presence/absence of MMR proteins and a PCR-based assay for microsatellite length polymorphisms. A normal IHC result is defined by the intact nuclear expression of MMR proteins, of which four are commonly tested: MLH1, MSH2, MSH6, and PMS2.9 A typical PCR panel examines five microsatellite markers, and samples with alterations in 40 percent (two or more) of these markers are resulted as MSI-high (MSI-H), whereas those with alterations in less than 40 percent (a single marker) are resulted as MSI-low (MSI-L), and those without any alteration are resulted as microsatellite stable (MSS).10 As changes in microsatellite length are a molecular manifestation of MMR protein dysfunction, the two tests are highly concordant.11 Per the NCCN, either IHC or PCR is an acceptable screening method for MMR status,8 although many institutions elect to perform both tests. Here, we report a case of a patient with colon cancer whose IHC and PCR tests returned discordant results, discuss the pros and cons of different testing methods, and explain what the discordant results might mean with respect to possible etiologies and clinical interpretation.
Case. The patient, a 62-year-old Caucasian male, presented to the emergency room with a five-month history of shortness of breath and intermittent dark tarry stools. Initial workup revealed anemia (Hgb 5.4 g/dL) and a positive fecal occult blood test. The patient’s family history was notable for colon cancer in his mother and two other maternal relatives, a maternal cousin with polyposis and a maternal grandmother who died of metastatic cancer of unknown primary. He had two abnormal colonoscopies nine and 10 years prior, for which he was offered surgical therapy but declined (prior histopathology unknown to authors).
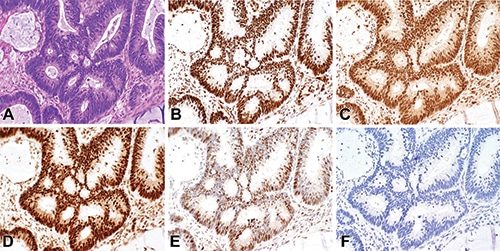
Fig. 1. Mismatch repair testing by immunohistochemistry demonstrates intact expression of DNA mismatch repair proteins in the neoplastic cells. A H&E; B MLH1; C PMS2; D MSH2; E MSH6; and F IHC negative control. Photomicrographs were taken with a 40 × magnification objective.
A diagnostic colonoscopy revealed a 9-cm ulcerated splenic flexure mass with invasive adenocarcinoma. Complete resection of the mass one month later identified a mucinous colonic adenocarcinoma with invasion into the subserosal adipose tissue and two lymph nodes positive for metastasis (pT3 N1b). Tissue from the resection specimen was submitted for MMR-deficiency testing by IHC and MSI testing by PCR, which returned discordant results. The IHC test showed retained expression of MLH1, MSH2, MSH6, and PMS2 in the neoplastic cells (Fig. 1). However, the PCR analysis identified MSI in five of five mononucleotide repeat alleles, an MSI-high result (Fig. 2). Despite the normal IHC result, the abnormal PCR result was deemed sufficient evidence of defective mismatch repair. The suspicion for a germline mutation was high because sporadic colorectal cancers with defective MMR typically show high MSI with concurrent loss of MLH1 and PMS2 by IHC.3 Thus the patient’s IHC and PCR results in combination with his strong family history of malignancy prompted referral for genetic counseling for further risk management for the patient and his family members.
The genetic counselor, in accordance with the testing strategies outlined by the NCCN,8 recommended germline testing as well as next-generation sequencing of the tumor to aid in identifying mutation-specific therapies and potential clinical trials. Germline testing identified a heterozygous pathogenic mutation in the MSH6 gene, c.1634_1635delAA, which causes a frameshift mutation with loss of function and premature protein truncation. This finding was diagnostic of Lynch syndrome. The NGS panel identified 13 somatic alterations in the tumor, including mutations in KRAS, APC, and TP53. No reportable alterations were identified in MLH1, MSH2, PMS2, EPCAM, or BRAF.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
