Diane Davis Davey, MD, Diane Davis Davey, MD, Ritu Nayar, MD
February 2014—The Cytopathology Education and Technology Consortium in 2009 issued a statement on human papillomavirus DNA test utilization that was published in multiple journals.1 This statement was a concise summary of the clinical indications for high-risk or oncogenic HPV testing based on guidelines of the American Society for Colposcopy and Cervical Pathology and the American Cancer Society published from 2002 through 2007.2,3 These organizations have since published newer consensus guidelines addressing HPV testing,4,5 and the previous summary no longer reflects current screening and management guidelines.
High-risk HPV testing has proven utility in cervical cancer screening and management. The 2012 screening guidelines endorsed by the ACS, ASCCP, and American Society for Clinical Pathology state that combined cervical cytology and HPV testing is now the preferred strategy for women age 30 and older. The 2012 ASCCP guidelines for management of abnormal cervical cancer screening tests and cancer precursors use co-testing extensively as a sensitive and efficient way to manage and follow these women. Inappropriate or too frequent screening, including HPV testing, can lead to increased costs without proven benefit and may cause patient harm by overtreatment. The following educational statement is intended to improve adherence to current guidelines, thereby improving the health care of women. The American Congress of Obstetricians and Gynecologists affirms these recommendations, and the U.S. Preventive Services Task Force says co-testing is acceptable.6,7
1. High-risk (oncogenic) HPV DNA testing is appropriate in the following circumstances.
1.1 Routine cervical cancer screening in conjunction with cervical cytology (co-testing) for women ages 30–65 years. (For women ages 30–65 with cytology reported as absent or insufficient endocervical/transformation zone component, early repeat cytology is not indicated and co-testing is preferred.)
1.1.1 For women whose cytology and HPV results are both negative, repeat both tests only after a five-year interval (applies only to routine screening; for women with negative co-tests following previous abnormal cytology, see below).
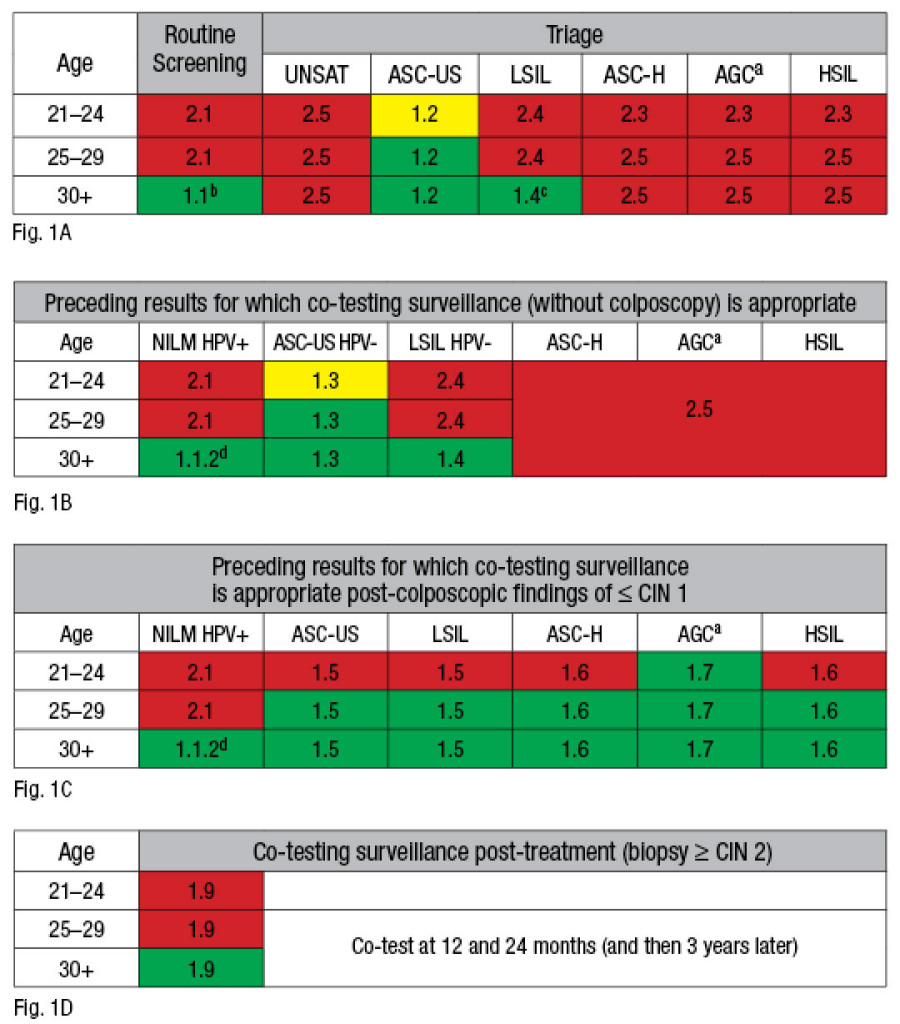 Fig. 1. Cell color indicates if HPV testing (or co-testing) is preferred (green), acceptable but not preferred (yellow), or not appropriate (red). Numbers in table cells refer to the text outline. Appropriate uses of human papillomavirus testing and co-testing (Papanicolaou test with HPV) are shown (A) in routine screening and triage, (B) as surveillance after abnormal Pap/HPV-positive (+) findings without colposcopy, (C) after colposcopy, and (D) after treatment. UNSAT indicates an unsatisfactory Pap test; ASC-US, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesion; ASC-H, atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion; AGC, atypical glandular cells; HSIL, high-grade squamous intraepithelial lesion; NILM, negative for intraepithelial lesion or malignancy; -, negative; CIN, cervical intraepithelial neoplasia.(a): For AGC results, HPV testing is not recommended as a triage tool; however, a negative HPV test may be helpful in suggesting endometrial versus endocervical origin. Co-testing is recommended at 12 and 24 months post-colposcopy.
Fig. 1. Cell color indicates if HPV testing (or co-testing) is preferred (green), acceptable but not preferred (yellow), or not appropriate (red). Numbers in table cells refer to the text outline. Appropriate uses of human papillomavirus testing and co-testing (Papanicolaou test with HPV) are shown (A) in routine screening and triage, (B) as surveillance after abnormal Pap/HPV-positive (+) findings without colposcopy, (C) after colposcopy, and (D) after treatment. UNSAT indicates an unsatisfactory Pap test; ASC-US, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesion; ASC-H, atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion; AGC, atypical glandular cells; HSIL, high-grade squamous intraepithelial lesion; NILM, negative for intraepithelial lesion or malignancy; -, negative; CIN, cervical intraepithelial neoplasia.(a): For AGC results, HPV testing is not recommended as a triage tool; however, a negative HPV test may be helpful in suggesting endometrial versus endocervical origin. Co-testing is recommended at 12 and 24 months post-colposcopy.(b): For women 30 and older who are both cytology and HPV negative, repeat both tests only after a five-year interval.
(c): For women 30 and older, HPV testing is preferred as a co-test, with repeat co-testing at one year if HPV is negative. HPV testing may be ordered as a triage for LSIL in postmenopausal patients.
(d): NILM/HPV+: Repeat both tests in one year (or perform HPV genotyping). Colposcopy is recommended if HPV positive on repeat regardless of cytology result.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
